

Pinterest. Sur Twitter : "Do you still pee in the swimming pool? You shouldn't, and here's why #chemistry #health... Sur Twitter : "Do you still pee in the swimming pool? You shouldn't, and here's why #chemistry #health... The Chemistry Behind the Smell of Wet Dogs #science #chemistry. Pin by Dani Valverde on Science. Biomedicine at the Turn of the Century. Simon Fan ✍ sur Twitter : "Crystallized protein up close. #Protein #Biochemistry #Science #Chemistry... Why Do Leaves Change Color? - Reactions. Así surgió la chispa de la vida a nivel atómico. Scientists Create Simple Artificial ‘Cell’ Capable Of Spontaneous Movement. The cells that make up all living things are in constant interaction with their environment.

Most cells perform complex chemical processes to ensure the cell and the organism remain healthy. Scientists have not yet been able to replicate a fully-functional synthetic cell, but it now appears they are off to a good start. Dynamic Periodic Table. First-ever high-resolution images of a molecule as it breaks and reforms chemical bonds.
May 30, Nanotechnology/Nanophysics Almost as clearly as a textbook diagram, this image made by a noncontact atomic force microscope reveals the positions of individual atoms and bonds, in a molecule having 26 carbon atoms and 14 hydrogen atoms structured as three connected benzene rings.

First-ever high-resolution images of a molecule as it breaks and reforms chemical bonds. When Felix Fischer of the U.S. Department of Energy's Lawrence Berkeley National Laboratory (Berkeley Lab) set out to develop nanostructures made of graphene using a new, controlled approach to chemical reactions, the first result was a surprise: spectacular images of individual carbon atoms and the bonds between them. "We weren't thinking about making beautiful images; the reactions themselves were the goal," says Fischer, a staff scientist in Berkeley Lab's Materials Sciences Division (MSD) and a professor of chemistry at the University of California, Berkeley. "But to really see what was happening at the single-atom level we had to use a uniquely sensitive atomic force microscope in Michael Crommie's laboratory. " Crommie is an MSD scientist and a professor of physics at UC Berkeley.
What the microscope showed the researchers, says Fischer, "was amazing. " The mysterious case of the missing noble gas. D.

Waldorf/Getty Who took the xenon? The evidence is in every breath of air, but answers are harder to come by. Xenon, the second heaviest of the chemically inert noble gases, has gone missing. Our atmosphere contains far less xenon, relative to the lighter noble gases, than meteorites similiar to the rocky material that formed the Earth. "Interstellar Space is a Quantum-Ruled Organics Lab" There may be a suite of organic chemical reactions occurring in interstellar space that astronomers haven't considered.

In 2012, astronomers discovered methoxy molecules containing carbon, hydrogen and oxygen in the Perseus molecular cloud, around 600 light years from Earth. But researchers were unable to reproduce this molecule in the lab by allowing reactants to condense on dust grains, leaving a mystery as to how it could have formed. Deconstruyendo la tabla periódica. Vitamin stops static electricity. A little vitamin E could zap static cling.
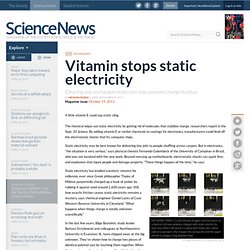
The chemical wipes out static electricity by getting rid of molecules that stabilize charge, researchers report in the Sept. 20 Science. By adding vitamin E or similar chemicals to coatings for electronics, manufacturers could fend off the electrostatic shocks that fry computer chips. Static electricity may be best known for delivering tiny jolts to people shuffling across carpets. But in electronics, “the situation is very serious,” says physical chemist Fernando Galembeck of the University of Campinas in Brazil, who was not involved with the new work. Beyond messing up motherboards, electrostatic shocks can spark fires and explosions that injure people and damage property.
NO MORE STATIC CLING Shreds of paper stick for hours to a block of silicone polymer charged with static electricity (top row). H.T. WATCH: Chemistry Made Seriously Cool! Direct observation of intermolecular interactions mediated by hydrogen bonding. A solvent's influence on a solute is of fundamental importance in chemical reactions 1 and is a ubiquitous feature of biomolecular processes, 2,3 and therefore, the study of solvation has a long and storied history.
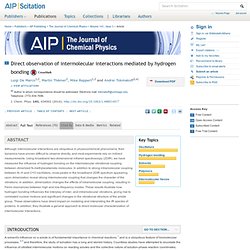
Directly visualizing hydrogen bonds. Using a newly developed, ultrafast femtosecond infrared light source, chemists at the University of Chicago have been able to directly visualize the coordinated vibrations between hydrogen-bonded molecules—the first time this sort of chemical interaction, which is found in nature everywhere at the molecular level, has been directly visualized.
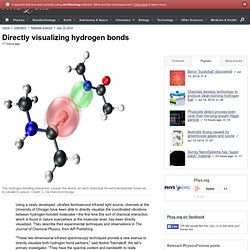
They describe their experimental techniques and observations in The Journal of Chemical Physics, from AIP Publishing. "These two-dimensional infrared spectroscopy techniques provide a new avenue to directly visualize both hydrogen bond partners," said Andrei Tokmakoff, the lab's primary investigator. Directly visualizing hydrogen bonds.
Jul 15, Chemistry/Materials Science The hydrogen-bonding interaction causes the atoms on each individual N-methylacetamide molecule to vibrate in unison.

Credit: L. De Marco/UChicago Using a newly developed, ultrafast femtosecond infrared light source, chemists at the University of Chicago have been able to directly visualize the coordinated vibrations between hydrogen-bonded molecules—the first time this sort of chemical interaction, which is found in nature everywhere at the molecular level, has been directly visualized. They describe their experimental techniques and observations in The Journal of Chemical Physics, from AIP Publishing. "These two-dimensional infrared spectroscopy techniques provide a new avenue to directly visualize both hydrogen bond partners," said Andrei Tokmakoff, the lab's primary investigator. Laser-Cooled Atoms: Ytterbium – Uncertain Principles.
The Chemistry of Fireworks. Fireworks are, as Dr John Cockling says in the video below, “chemistry in action”.
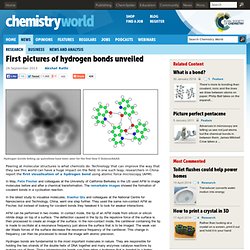
It takes 18 elements to provide the range of colors we see in a big display. However, while we are all for people finding loud and brightly colored ways to get inspired by science, please take care – injuries are common. We'd also encourage everyone to be sensitive to their neighbors, and particularly pets, whose hearing is often particularly affected by the noise of rockets shooting in air and the loud bangs that follow. The chemistry of fireworks is astonishingly complex. If the above video just whetted your appetite, here is a, shall we say, slower burning lecture on the chemistry required to put on those displays. First pictures of hydrogen bonds unveiled.
Hydrogen bonds linking up quinolines have been seen for the first time © Science/AAAS Peering at molecular structures is what chemists do.
Laser-Cooled Atoms: Ytterbium – Uncertain Principles.