

Why Do Oil and Water Not Mix. The Magic School Bus S01E09 Gets Ready, Set, Dough (Kitchen Chemistry)
4608762.jpg (JPEG Image, 236 × 303 pixels) Ice, Water and Oil Experiment - FUN Science for Kids! My kids are so interested and amazed with Science, they love learning first hand how things in their environment interact.
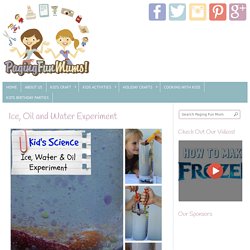
I also remember loving Science as a child, I vividly remember doing this Ice, Oil and Water Experiment in Science class in grade 7. Here’s what we used for our home experiment – A large glass Vase (you could do a smaller sized regular drinking glass)Baby Oil – enough to fill your vase 3/4 fullFrozen Ice cubes filled with water and food colouringtongsempty syringes or dopplers Directions – Carefully fill your glass vase, have your child hold the ice using the tongs in the oil mixture, then patiently wait and watch…you will slowly start to see a coloured drop of melted water start to bead off the ice and gracefully sink to the bottom. My 5 year old daughter found it hard to keep the slippery ice in the tongs so I grabbed some old medicine syringes, we filled these with coloured water, the kids had a great time squirting the water into the oil. How to Make Magic Aqua Sand. Wow!
This is such a FUN and AMAZING play product that you can make at home! The kids will spend hours playing with this Magic Aqua Sand! I would recommend it’s made by adults for the kids to use under supervision. Extra Credit Topics - Mrs. Roper's Chemistry Site. Introduction You have this awesome opportunity for extra credit in chemistry.

This is so you have every chance to improve your grade and make the grade you want! I encourage all of you to take charge of your grade. Please read the following information carefully and let me know if you have any questions. An example assignment is attached for you to reference. Chemistry extra credit options. What are trees made of?
Veritasium answers more questions about trees like: How can trees be so tall?
Go out on a limb and find out. Watch and learn the answer to this question! What is the world's tallest tree? Watch this National Geographic video and find out! Go along with scientists as they study, The President," one of the tallest trees in the world! What is the true value of a tree? Planting trees has restored depleted ecosystems and helped struggling communities worldwide. What exactly is meant by a closed system? What is the true monetary value of a tree? Interested in reading a book about the tallest trees in the world? Ss1809 34. Eighth Grade Physical and Chemical Properties, Chemical Change. All matter is formed from atoms of some 100 different elements that occur naturally within the Earth.
Each pure substance has predictable physical and chemical properties. In a system, atoms combine chemically with other atoms to regroup and form new molecules of matter with different properties than each atom’s original properties. Since matter cannot be created nor destroyed, when these chemical reactions occur, matter is conserved and the original number of atoms before a reaction occurs (product) is the same as the number of atoms after the reaction occurs (reactant). Just as matter is conserved, so too is energy. Students can utilize both of these concepts to construct explanations for how food is utilized for energy transfer in organisms.
Energy plays an important role in chemical reactions. To make application of these concepts, students can undertake a design project to construct, test, and modify a device that either releases or absorbs thermal energy by chemical processes. Engineering students create walk on... - Interesting Engineering. Spoon Mold-Make it Yourself! Gmail - Free Storage and Email from Google. Can You Copperplate? MS-PS1-2 Analyze and interpret data on the properties of substances before and after the substances interact to determine if a chemical reaction has occurred.
Clarification Statement: Examples of reactions could include burning sugar or steel wool, fat reacting with sodium hydroxide, and mixing zinc with hydrogen chloride. Assessment Boundary: Assessment is limited to analysis of the following properties: density, melting point, boiling point, solubility, flammability, and odor. This resource was not designed to build towards this performance expectation, but can be used to build towards it using the suggestions provided below.
Matter Matters Unit (Outline and storyline only) Free K - 12 Common Core Lesson Plans and Ideas. Middle School Physical Sciences Scroll Up.
Free K - 12 Common Core Lesson Plans and Ideas. Free K - 12 Common Core Lesson Plans and Ideas. Download Free Science Activities, Access Chemistry Multimedia, Find Information on Workshops. Chemistry. Happy Atoms. Link overview chemistrymodels. Conservation mass lab4. 4608762.jpg (JPEG Image, 236 × 303 pixels) Water Electrolysis. Middle School Chemistry Unit. Skip Navigation Middle School Chemistry big ideas about the very small Lessons Materials Vist the materials page to see exactly which materials you'll need to complete the lessons in Chapter 6.
Teaching Resources Student Reading Student Reading for chapter 6 Teacher Background Test Bank Questions Chapter 6 Sample Multimedia Molecules Collidefrom Chapter 6, Lesson 4 Combustion of Methanefrom Chapter 6, Lesson 1 Balanced Equationfrom Chapter 6, Lesson 1 Methane and Oxygen Reactfrom Chapter 6, Lesson 1. Delta Education FOSS Middle School Complete Courses - FOSS Middle School Chemical Interactions.
Energy Forms and Changes - Energy, Conservation of Energy, Energy Transfer. Atoms Bonding Song. Download Free Science Activities, Access Chemistry Multimedia, Find Information on Workshops. Download Free Science Activities, Access Chemistry Multimedia, Find Information on Workshops. Water Electrolysis. Cf10371.pdf. Seventh grade Atomic Structure and Bonding Lessonplans, homework, quizzes. Seventh grade Atomic Structure and Bonding Seventh gradeEighth Grade 1 more ... 1,981 Views 2 Favorites periodic-table-shells-valence.pptx Kenna Patterson from Clarksville Junior High School Location: 9.20 Neutrons, Protons, Electrons Description: Put this on Smart Board and had students follow along.
91419 Reaction in a Bag - 91419.pdf.