

cyrus poonawalla group
Cyrus Poonawalla Group is among India's top private sector business houses serving over millions of people across financial services, vaccine manufacturing, pharmaceutical, renewable energy, real estate & hospitality sectors
Bio Waste Management Company. Poonawalla Clean Energy’s focus on environmental sustainable solutions and technology to meet the rapidly growing need of renewable energy has recently resulted in the funding of several companies who’s core objectives are renewable resources of energy.

Noble Exchange Environment Solutions (NEX) founded in 2011 and recently funded by the Cyrus Poonawalla Group is a Bio waste management company specializing in processing of Organic Food Waste into BioGas and Organic Manure as its by-products. Biogas when cleaned and purified to 96% purity is called Compressed Bio Gas (CBG) and can be used as alternate fuel for natural gas vehicles or replace other fossil fuels such as LPG, Diesel, Furnace Oils, etc. Biogas is considered to be a renewable resource because its production-and-use cycle is continuous and it generates no net carbon dioxide.
Poonawalla Aviation Private Limited. Financial Services Company in India. Leading vaccine manufacturer in india. Serum Institute of India is the world's largest vaccine manufacturer by number of doses produced and sold globally (more than 1.5 billion doses) which includes the Polio vaccine as well as Diphtheria, Tetanus, Pertussis, Hib, BCG, r-Hepatitis B, Measles, Mumps and Rubella vaccines.

Founded in 1966 by Dr. Cyrus Poonawalla with the aim of manufacturing life-saving immuno-biologicals, SII is now ranked as India's No. 1 biotechnology company, It is estimated that about 65% of the children in the world receive at least one vaccine manufactured by Serum Institute. Vaccines manufactured by the Serum Institute are accredited by the World Health Organization (WHO) and are used in their national immunization programs across the world. Serum Institute's commitment to global health is exemplified by significant investments in its infrastructure. Cyrus Poonawalla Group of industries India. SERUM INSTITUTE OF INDIA TO PRODUCE UP TO 100 MILLION COVID-19 VACCINE DOSES FOR INDIA AND LOW-AND MIDDLE-INCOME COUNTRIES AS EARLY AS 2021.
07 August, 2020 New landmark collaboration between the Serum Institute of India (SII), Gavi, and Bill & Melinda Gates Foundation.

The Bill & Melinda Gates Foundation, via its Strategic Investment Fund, will provide at-risk funding of US$150 million to Gavi, which will be used to support the SII to manufacturethepotential vaccine candidates. Vaccines will be priced at maximum US$3 per dose and made available to the 92 countries included in Gavi’s COVAX Advance Market Commitment (AMC). India, 7th August 2020 – Serum Institute of India (SII), the world’s largest vaccine manufacturer by volume, has entered into a new landmark partnership with Gavi, The Vaccine Alliance and the Bill & Melinda Gates Foundation, to accelerate the manufacture and delivery of up to 100 million doses of COVID-19 vaccines for India and low-and middle-income countries (LMICs). Dr. Bilthoven Biologicals: Bio-Engineering & Pharmaceutical Company. Poonawalla Real Estate and Hospitality. Villoo Poonawalla Greenfield Farms. Established as the Poona Stud Farm in 1946 by the late Soli A.
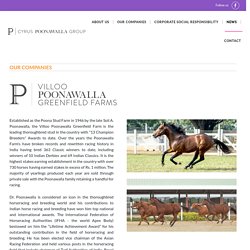
Poonawalla, the Villoo Poonawalla Greenfield Farm is the leading thoroughbred stud in the country with "13 Champion Breeders" Awards to date. Over the years the Poonawalla Farm’s have broken records and rewritten racing history in India having bred 363 Classic winners to date, including winners of 10 Indian Derbies and 69 Indian Classics. It is the highest stakes earning establishment in the country with over 730 horses having earned stakes in excess of Rs. 1 million. The majority of yearlings produced each year are sold through private sale with the Poonawalla family retaining a handful for racing. Dr. The Poonawalla family continue to maintain their passion for thoroughbred horses and sponsor the most glamorous event in Indian horse racing calendar – Poonawalla Breeder’s Multi Million, the richest juvenile race in the country. Visit Farm Website. Bio Waste Management Company.
Poonawalla Aviation Private Limited. Financial Services Company in India. Leading vaccine manufacturer in india. Cyrus Poonawalla Group of industries India. SERUM INSTITUTE OF INDIA CEO: ‘WE PLAN TO MAKE MILLIONS OF DOSES OF COVID VACCINE OVER THREE MONTHS’:’ Serum Institute of India Pvt Ltd, the world’s largest vaccine manufacturer by the number of doses produced and sold globally (more than 1.5 billion doses), has partnered with AstraZeneca to support efforts for a vaccine against Covid-19.
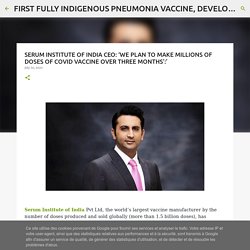
It has also got the DCGI go-ahead to manufacture its own indigenously developed pneumococcal vaccine. Serum Institute CEO Adar Poonawalla speaks to Anuradha Mascarenhas about their efforts to develop these vaccines. How long will it take for a vaccine against Covid-19 to be made available? Serum Institute of India gets approval to start human trials for Oxford vaccine. 01 August, 2020 Earlier this week, a committee of experts had deferred a decision on the request of SII to start trials and asked the company to amend its protocol for the clinical study.

The subject expert committee (SEC) had recommended eight amendments to be made to the firm's proposal to conduct Phase-II and -III trials. NEW DELHI: The Serum Institute of India (SII) got the approval on Friday to start human trials in India for the Covid-19 vaccine candidate developed by University of Oxford, after the company submitted a revised protocol to the drug regulatory authority, people in the know told ET. On Wednesday, a committee of experts had deferred a decision on the request of SII to start trials and asked the company to amend its protocol for the clinical study.
The subject expert committee (SEC) had recommended eight amendments to be made to the firm's proposal to conduct Phase-II and -III trials. For further information visit - The Economic Times. Serum Institute conducting phase III clinical trial of BCG vaccine: DBT. 27 July, 2020 About 6,000 health workers and high-risk individuals including those in close contact of COVID-19 patients have been enrolled in a clinical trial to determine if the recombinant Bacillus Calmette-Guerian (rBCG) can boost immunity to fight against the virus, the statement added.
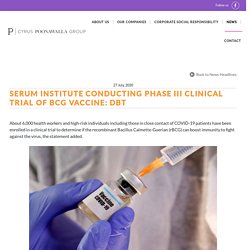
The Serum Institute of India is conducting phase III clinical trial of BCG vaccine candidate VPM1002 to evaluate its ability in reducing infection and severe disease outcomes of COVID-19 among high-risk persons of advanced age, comorbidities and high-exposure healthcare workers (HCWs), the Department of Biotechnology said in a statement. The BCG vaccine is administered routinely to all newborns as part of the national childhood immunisation programme to prevent tuberculosis (TB), an infection caused by bacteria that mainly affects the lungs. "The trial began in May 2020 and has completed enrolment of 6,000 subjects in almost 40 hospitals across the country. For further information visit - MoneyControl. Leading vaccine manufacturer in india. Bio Waste Management Company.
Poonawalla Aviation Private Limited. Financial Services Company in India. Leading vaccine manufacturer in india. Cyrus Poonawalla Group of industries India. Poonawalla Aviation Private Limited. Financial Services Company in India. Financial Services Company in India. Leading vaccine manufacturer in india. Julia Chatterley In Conversation with CEO Mr. Adar Poonawalla. Sabina Sanghvi in Conversation with Dr. Cyrus Poonawalla. Bio Waste Management Company. Poonawalla Aviation Private Limited. Poonawalla Aviation Private Limited. Financial Services Company in India. Leading vaccine manufacturer in india. Bilthoven Biologicals: Bio-Engineering & Pharmaceutical Company. Poonawalla Real Estate and Hospitality. Villoo Poonawalla Greenfield Farms.
50% of our Covid vaccines will be for India, people won't have to buy them, govts will pay: Adar Poonawalla. 22 July, 2020.
Cyrus Poonawalla Group of industries India. AstraZeneca vaccine's success in early human trials, Will Begin AstraZeneca Vaccine Trial in India After Applying for Licence Next Week, Says Serum Institute. 21 July, 2020 SII, the largest vaccine manufacturer in the world, has been chosen by Oxford and its partner AstraZeneca to manufacture the vaccine once it gets ready.
With the trials of COVID-19 vaccine being developed by AstraZeneca and Oxford University showing encouraging results, Serum Institute of India (SII) on Monday said it will apply for licence from the Indian regulator to start clinical trials of the shot in a week's time. SII, the largest vaccine manufacturer in the world, has been chosen by Oxford and its partner AstraZeneca to manufacture the vaccine once it gets ready. Earlier, Pune-based SII had said that it will start manufacturing the vaccine even before the final nod so as to be ready with sizeable volumes once the vaccine gets all permissions. "The trials have shown promising results and we are extremely happy about it. "In addition, we will soon start manufacturing the vaccine in large volumes," SII Chief Executive Officer Adar Poonawalla said. ASTRAZENECA VACCINE'S SUCCESS IN EARLY HUMAN TRIALS, WILL BEGIN ASTRAZENECA VACCINE TRIAL IN INDIA AFTER APPLYING FOR LICENCE NEXT WEEK, SAYS SERUM INSTITUTE.
With the trials of COVID-19 vaccine being developed by AstraZeneca and Oxford University showing encouraging results, Serum Institute of India (SII) on Monday said it will apply for licence from the Indian regulator to start clinical trials of the shot in a week's time.

SII, the largest vaccine manufacturer in the world, has been chosen by Oxford and its partner AstraZeneca to manufacture the vaccine once it gets ready. Earlier, Pune-based SII had said that it will start manufacturing the vaccine even before the final nod so as to be ready with sizeable volumes once the vaccine gets all permissions. "The trials have shown promising results and we are extremely happy about it. SERUM INSTITUTE OF INDIA CEO: ‘WE PLAN TO MAKE MILLIONS OF DOSES OF COVID VACCINE OVER THREE MONTHS’:’ Leading vaccine manufacturer in india. Cyrus Poonawalla Group of industries India.
Serum Institute of India CEO: ‘We plan to make millions of doses of Covid vaccine over three months’ 18 July, 2020 Serum Institute CEO Adar Poonawalla speaks to The Indian Express about their efforts to develop Covid-19 vaccines Serum Institute CEO Adar Poonawalla Serum Institute of India Pvt Ltd, the world’s largest vaccine manufacturer by the number of doses produced and sold globally (more than 1.5 billion doses), has partnered with AstraZeneca to support efforts for a vaccine against Covid-19. It has also got the DCGI go-ahead to manufacture its own indigenously developed pneumococcal vaccine. Serum Institute CEO Adar Poonawalla speaks to Anuradha Mascarenhas about their efforts to develop these vaccines.
How long will it take for a vaccine against Covid-19 to be made available? First Fully Indigenous Pneumonia Vaccine, Developed By Serum Institute, Approved. Serum Institute of India expects COVID vaccine by year-end. 8 July, 2020 He was speaking during the launch of 'Compact XL, a compact diagnostics machine by MyLab Discovery Solutions, that will automate lab processes from sample handling to preparing RT-PCR tubes Vaccine maker Serum Institute of India (SII) is hoping to develop a COVID-19 vaccine by the year-end as it is focusing on a “good and safe” product and is not in a “rush”, the company’s CEO Adar Poonawalla said. He was speaking during the launch of ‘Compact XL, a compact diagnostics machine by MyLab Discovery Solutions, that will automate lab processes from sample handling to preparing RT-PCR tubes. Covid-19 Vaccine devloped by Oxford University with Serum Institute of India gone into Human Trial in UK- Adar Poonawalla.