

What the judge got wrong in the transvaginal mesh device scandal. Last November, three women, representing thousands of patients given defective vaginal mesh implants, won a landmark class action against Johnson & Johnson.
The Federal Court of Australia ruled that the company had misled customers about the risks linked to the devices, and was liable for the injuries caused to the women resulting from the surgery they underwent. But, in the aftermath, doctors have raised concerns over whether the Federal Court judgement misunderstood some of the clinical evidence, specifically for the use of the devices in the treatment of urinary stress incontinence. Here, Professor Chris Maher, one of Australia's leading urogynaecologists, explains. Skynews.com. Where were the regulators? How authorities failed pelvic mesh victims. Ethicon wasn't ignorant of the fact it might need to treat women's pelvises with care.
It just didn't do so. Some time after it released one of its largest, most invasive and injurious devices, the Prolift, which was on the Australian market in 2005, it acknowledged a problem internally and confidentially. Related Article "Today's vaginal implants do not consider the patients' biomechanical needs" which could, and did, lead to "misfunction, pain and shrinkage" and ultimately a "handicapped patient", an Ethicon research and development team acknowledged. But rather than pull Prolift from the market Ethicon continued to spruik it at doctors' conferences around the world, and developed a revised device in an attempt to address the problems, which it released with "little clinical evidence".
It did consider designing a mesh device specifically adapted to a woman's pelvic floor but, as Katzmann found, "nothing eventually came of it and the project was abandoned in 2011". Development in landmark class action against makers of defective vaginal mesh implants. Three women representing thousands of patients given defective vaginal mesh implants have won a landmark class action against international medical giants.
The Federal Court on Thursday found two Johnson & Johnson group manufacturers and its Australian supplier engaged in misleading or deceptive conduct and hid the reality of complications from customers. More than 90,000 devices were inserted into those wanting structural support to treat stress urinary incontinence and pelvic organ prolapse. But hundreds, if not thousands, who elected to undergo surgery suffered chronic and debilitating acute pain as the supposedly permanent implants eroded. More on 7NEWS.com.au One of those in the class action, Victoria's Diane Dawson, remains beset by pain despite five additional surgeries to address her complications, Justice Anna Katzmann SC said. Skynews.com. Newcastleherald.com. News, local-news, MORE than 1200 Australian women have won their landmark class action legal case against pharmaceutical giant Johnson & Johnson and related Ethicon companies after they experienced severe and permanent injuries following pelvic mesh surgery.
Federal Court Justice Anna Katzmann has told a packed courtroom that evidence was "overwhelmingly" against the companies after some women experienced "disastrous" results. In a scathing commentary Justice Katzmann has slammed Johnson & Johnson and related companies Ethicon Inc and Ethicon Sarl for the release of nine pelvic mesh devices from 1999 with little evidence supporting their safe use. The companies minimised known complications for years, regarded risk management assessments as a largely "tick a box exercise", and failed post-marketing monitoring despite growing evidence of serious injuries to women, Justice Katzmann said. The Ethicon devices were "oversold" and carried significant risks, Justice Katzmann said. Vaginal mesh surgery complications left Linda Schultz suicidal. The loss of a life well lived research exploring the impact of surgical mesh implants on a group of new zealand women october 2019.
The 'eight-minute' cure: how transvaginal mesh sentenced thousands of women to a life of pain. Grace Irvine has blue eyes, pale skin, and short hair that fluffs up at the back like a baby chick's.
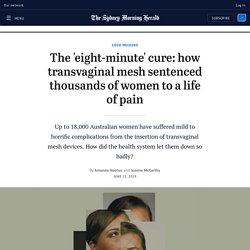
She is 29 years old. Three years ago, she was a healthy mother of three boys living in a small town in Victoria. She worked as a dental assistant, and wanted to study nursing. After her second son, Sammy, was born in 2013, Irvine noticed what felt like an uncomfortable lump in her vagina when she sat down. This is a common symptom of pelvic organ prolapse (POP), a condition that, at some point, affects about half of all women who've had children. "I didn't want to be wearing nappies for the rest of my life," she explains, smiling. Australian-doctor-face-of-international-clinical-trial-for-controversial-pelvic-mesh-20190820-p52isl. Following questions from The Sydney Morning Herald, Dr Petros said the trial had been withdrawn from the Australian New Zealand Clinical Trials Registry (ANZCTR) for "logistic reasons".
But on Tuesday night the trial was still lodged on the ANZCTR website with Dr Petros listed as the public contact. Dr Petros said via email he has "never been a participant of the trial" but he did help with the registration. Earlier this year in the NSW Civil and Administrative Tribunal case, the Health Care Complaints Commission (HCCC) alleged Dr Petros had surgically implanted the TFS device in 108 patients between 2013 and 2015 and failed to disclose that he and his family had a financial interest in the product.
Despite the Therapeutic Goods Administration (TGA) cancelling the device’s registration in November 2014, it was still being used in some surgeries at The Sydney Private Hospital. Days earlier on May 23, the Austrian trial involving Dr Petros was submitted to the ANZCTR. Related Article. UC 332: Pelvic Mesh Implants Gone Wrong with Justine Watson - The Wellness Couch. Justine Watson President & Co-Founder of Mesh Injured Australia had a TVT fitted in 2010 for SUI.
She discovered in 2017 after a cystoscopy that the mesh had skewered her urethra in several places and was embedded in her bladder. With no other explanations for her years of pain and systemic illnesses and after much researching, she travelled alone to the USA for removal surgery a few weeks later. Justine is a mental healthcare worker with more than 20 years experience and is now an advocate for Mesh Injured people and is currently involved in the co-design of a new Pelvic Mesh clinic in Sydney.
The new robotic surgery aiding vaginal mesh removal. Gai Thompson says governments have failed to hold doctors and regulators to account for the pelvic mesh scandal. News, local-news, GAI Thompson addressed an email to her local MP before she drained her superannuation fund and flew to America on Mother's Day to have pelvic mesh devices implanted in 2008 removed.

In the email Mrs Thompson said she wanted to gain relief from "this mesh that is destroying my health, mind and body because I truly can't live like this anymore". "My hope is that I could arrange an appointment with your office to discuss what could be done for all mesh injured women... to hold the pharmaceutical companies, Therapeutic Goods Administration and surgeons responsible for what they have done," she wrote.
Mrs Thompson, 55, received a sympathetic email in response, with a copy of Health Minister Greg Hunt's apology in October, 2018 to thousands of Australian mesh-injured women, in which he acknowledged "horrific outcomes" for some. Surgical mesh ban campaigner: 'It's plastic and will eventually break down' A Christchurch retiree fought for for two years to have surgical mesh removed after a hernia repair procedure that left him in agony.
Now, he wants the controversial procedure banned. John Pritchard, 74, says his life was ruined by the June 2016 surgery, when his body rejected the mesh causing constant pain in his groin. Diagnosed with a strangulated hernia – when the contents of the abdomen protrude through an area of weakened stomach wall and threaten to block blood supply – he was booked for surgery the following day. READ MORE: * Grandma first spoke about surgical mesh five years ago; today, she is still suffering * More surgical mesh injuries denied ACC cover * Using surgical mesh for hernias 'safe and effective', surgeon says * Four mesh deaths but government drags heels on inquiry: 'Mum always put on a brave face for us' * Hernia mesh complication 'like torture' for Christchurch grandfather "I just want to make sure no one else goes through what I went through," he said.
Transvaginal mesh: Young mum against Johnson and Johnson. 1300 women are standing against Johnson & Johnson for the manufacture and distribution of transvaginal mesh.
“It will be like having a designer vagina,” Rebecca Oates recalls the surgeon saying, before she was anaesthetised and had transvaginal mesh inserted through her vagina, looped around her urethra and pulled tight over her pubic bone. Only 27, Oates had just had her second child and was experiencing prolapse and stress incontinence. "I was unaware at the time but this product was about to completely destroy who I was as a mother, wife, friend, employee, and woman in general and I wish to god every day since that I would have never gone ahead with the surgery," Rebecca has since said. Now 32, Oates is one of approximately 1300 who have registered to take part in action against major pharmaceutical brand, Johnson & Johnson, the manufacturers and distributors of transvaginal mesh.
Rebecca's life was turned upside down after the surgery. Making history. Forums planned to hear from New Zealanders affected by surgical mesh. The Ministry of Health's Director-General Dr Ashley Bloomfield has welcomed the chance for New Zealand men and women harmed by surgical mesh to participate in restorative justice forums. “Forums will be held around the country from later this month to hear from people affected by surgical mesh, and I invite people to register now to tell their story. “Complications from surgical mesh have caused a considerable amount of pain and suffering for a significant group of people and I have a huge amount of sympathy for every person affected. "We have already taken steps to limit the use of surgical mesh and improve patient safety.
Now we want to hear directly from those who have been harmed and this will guide further action needed to address the ongoing impact on people affected and prevent future harm. “Earlier this year we ran an online survey, which had over 400 responses, where people told us about the importance of establishing these forums. Woman who suffered 'dreadful pain' after surgical mesh implant, welcomes restorative justice forums. The 'eight-minute' cure: how transvaginal mesh sentenced thousands of women to a life of pain. Mesh Injured Australia's President Justine Watson talks with ABC NewsBreakfast. Theherald.com. News, local-news, AMERICAN regulators have banned a problem group of pelvic mesh devices nearly 18 years after they appeared on the market, and as the Australian doctor who invented one of the first of the US-approved problem devices faces professional misconduct proceedings in NSW.
The American Food and Drug Administration (FDA) has ordered all remaining manufacturers to immediately stop selling and distributing prolapse mesh devices because of safety and efficacy concerns, and after billions of dollars in damages claims by thousands of women in America, Australia, the United Kingdom and Europe. The ban comes 18 years after the FDA approved an Australian-invented mesh device to treat prolapse, a complication after childbirth, only months after Australian reviewers in February, 2001 found no "good quality evidence to determine the safety and efficacy" of the device procedure. A Senate inquiry in 2017 was told about 150,000 prolapse and incontinence devices have been sold in Australia. US Food and Drug Administration halts pelvic organ mesh sales. The US Food and Drug Administration (FDA) has ordered an immediate halt to the sale of surgical mesh products to treat pelvic organ prolapse (POP).
The FDA said the action this week was taken as two remaining manufacturers of the product, Boston Scientific and Coloplast, had not been able to prove the products were safe to treat pelvic prolapse – when the uterus bulges through weakened tissue into the vagina. Ministry of Health chief medical officer Dr Andrew Simpson welcomed the FDA decision. Joan Isaacs gave evidence against the Catholic Church while struggling after pelvic mesh surgery. Newsletters, editors-pick-list, A CATHOLIC Church child sexual abuse survivor who prayed for death to relieve extreme pain after pelvic mesh surgery has accused key elements of Australia's health system of acting like the church over the mesh scandal.
"I went to the Catholic Church and there was minimising, inaction and denial. I complained after mesh surgery and the response was the same from the health system. It was just denying, denying, denying there was a problem with mesh for years," said Joan Isaacs, who was one of the first Catholic survivors to give evidence to the child abuse royal commission in 2013. OPINION: We accept product failure in our health system that we wouldn't consider in cars or food, says consumer advocate. Mesh destroys 'intimacy in marriage' but Kiwi victim cannot sue.
A woman, so damaged from vaginal mesh her husband was advised to wear two condoms to prevent being cut during sex, cannot sue the manufacturer because of ACC. Jan*, 58, says the mesh has all but destroyed the intimacy in her marriage. "It's affected me a lot because if my husband and I go to have relations it's always … I'm thinking am I hurting him? "At one stage the doctor suggested we use double condoms and you know we're in our 50s and we're not interested in doing that.
" Govt to mesh victims: "tell us your stories" 13 DHBs no longer doing mesh surgery for incontinence. Theherald.com. Pelvic mesh implants: Further legal action on the table after scathing Senate report - ABC News (Australian Broadcasting Corporation) Updated 29 Mar 2018, 8:19amThu 29 Mar 2018, 8:19am. Pelvic mesh: Obstetrician says profession must learn from scandal, calls for register - ABC News (Australian Broadcasting Corporation) Posted about 10 hours agoTue 23 Oct 2018, 10:04pm. British mesh investigation paints 'hair raising' picture of 'scandal' Grandmother living in agonising pain from mesh 'life sentence' calls for apology.
Doctored: mesh surgeon Richard Reid admits to writing fake patient notes. Mesh implants: Government issues national apology over 'agony and pain' caused by device. Updated about an hour agoTue 9 Oct 2018, 9:49pm Health Minister Greg Hunt has issued a national apology to the many women whose lives have been ruined by the debilitating effects of pelvic mesh implants. Key points: Auckland woman dead after two years of pain from surgical mesh. The Medical RepublicThe Medical Republic. Women are in danger of losing a perfectly safe and effective device for urinary incontinence, as the scandal over transvaginal mesh catches mid-urethral slings in its wake.
Early last year the Senate in Canberra launched an inquiry in response to women coming forward with reports of serious side-effects from mesh implants. While many have welcomed the opportunity to address problems around mesh, leading gynaecologists expressed concern at last week’s RANZCOG Annual Scientific Meeting in Adelaide that the commission had bundled slings into the same category.
“There’s not a gynecological operation that’s been studied more than mid-urethral slings for stress incontinence,” former AMA president Dr Michael Gannon told The Medical Republic. Theherald.com. WOMEN have accused the Australian Government of standing by while devastated pelvic mesh patients are re-traumatised by a health system failing to acknowledge and respond to a national scandal.
Standard improved to stop 'tragic' surgical mesh stories. Open letter from Christine Rankin regarding surgical mesh. Vaginal mesh implants: Gynaecologist urges proactive response to health concerns. A-weak-pelvic-floor-can-mean-more-than-a-little-leakage-20180605-p4zjje. The biggest lesson from the transvaginal mesh saga? Doctors must listen to women.
Second pelvic mesh surgeon faces professional misconduct hearing. Pelvic mesh implants: How tracking vehicle parts is easier than medical devices. New report: a damning indictment of health system’s failure to care for women. MIA women bring pain and suffering to light to save others from same trauma. Doctored: mesh surgeon admits to writing fake patient notes. Son’s fears for mother ‘broken’ by mesh surgery. Johnson & Johnson accused of failing to warn patients at higher risk from vaginal mesh. Australia bans mesh after Scots’ evidence. Horror Stories From The Australian Pelvic Mesh Implant Report. Australia investigates implants that left some women with 'rotting pelvises' Vaginal mesh: Senate hears hundreds of women damaged for life. Vaginal mesh only as last resort: report. Pelvic mesh implants 'one of the biggest medical scandals' involving Australian women.
Pelvic mesh implants: How tracking vehicle parts is easier than medical devices. Australian women join legal actions over pelvic implants. Surgical mesh patients 'totally failed' by health system, gynaecologist says. Johnson & Johnson Has Withdrawn The Mesh Products It Is Being Sued Over. Johnson & Johnson Knew Its Vaginal Mesh Implants Could Erode, Lawyer Argues. Johnson & Johnson withdraws pelvic mesh device from Australian market. The ‘cruellest thing of all’: daughter’s shock over missed pelvic mesh audit calls. Australian women’s grief after mother’s death linked to pelvic mesh. Johnson & Johnson pelvic mesh doctor said he would not want his wife to undergo procedure, Federal Court told. Derryn Hinch’s grim warning after suicide of pelvic mesh victim. The Stories Of Women Who Have Had Mesh Implants Are Heartbreaking.
Woman’s death after pelvic mesh complications: ‘The pain is just too much’ Grandma first spoke about surgical mesh five years ago; today, she is still suffering. Victim 'overwhelmingly emotional' after decision to limit use of surgical mesh that left hundreds in 'awful chronic pain' New Zealand becomes first country to fully ban controversial vaginal mesh procedures. NZ women without options after ban on all mesh products. Doctors say blanket restriction of surgical mesh not the way to go. New Zealand is Second in World to Restrict the Use of Surgical Mesh. Without warning, Australia bans surgical mesh products for pelvic organ prolapse.
Australia bans use of vaginal mesh implants. The medical device implanted into hundreds of women which is now no longer legal to use - Lateline. Theherald.com. Vaginal mesh scandal in the UK could be 'bigger than thalidomide' Surgical mesh injuries rising: ACC. Mesh, money and medicine. Medical Council stops Reid from doing major surgery. Helper seeks help over mesh ‘suffering’
More surgical mesh injuries denied ACC cover. Theherald.com. Life after mesh surgery at 22. Health Ministry looks at establishing mesh devices register. The Stories Of Women Who Have Had Mesh Implants Are Heartbreaking. News Item. Perth doctor 'aghast' at sex advice to pelvic mesh victims. Australian Medical Association president confirms AMA WA's role in pelvic mesh scandal.
Explaining the vaginal mesh controversy - Faculty of Medicine - The University of Queensland, Australia. Lamborghinis, ski trips used to market controversial mesh implant to surgeons, documents show. Lateline - 14/08/2017: Lamborghinis, ski trips used to market mesh implants to surgeons, documents show. Potentially damaging surgical mesh kits still on hospital shelves with doctors unaware of cancellation.
A Lawyer Who Won $20 Million For His Client Says We Are Facing "The Greatest Women's Health Crisis Of Our Generation" Emails show doctors' bizarre attitudes to women's health. Hinch predicts mesh ‘cover-ups’ Perth women 'used as pelvic mesh guinea pigs' want answers from WA government. It’s ‘first do no harm’ Vaginal mesh risks downplayed by Johnson & Johnson, court told. Vaginal mesh implants: Gynaecologist urges proactive response to health concerns.