

Electroactive polymers. (a) Cartoon drawing of an EAP gripping device.
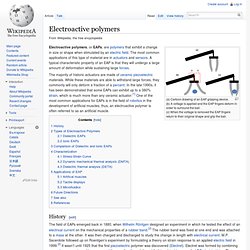
(b) A voltage is applied and the EAP fingers deform in order to surround the ball. (c) When the voltage is removed the EAP fingers return to their original shape and grip the ball. Electroactive polymers, or EAPs, are polymers that exhibit a change in size or shape when stimulated by an electric field. The most common applications of this type of material are in actuators and sensors.
A typical characteristic property of an EAP is that they will undergo a large amount of deformation while sustaining large forces. History[edit] The field of EAPs emerged back in 1880, when Wilhelm Röntgen designed an experiment in which he tested the effect of an electrical current on the mechanical properties of a rubber band.[2] The rubber band was fixed at one end and was attached to a mass at the other.
Polymers that respond to environmental conditions other than an applied electrical current have also been a large part of this area of study. [edit] Conductive Polymers – The Lubrizol Corporation. Clean Room Stat-Rite® IDP alloys deliver permanent electrostatic dissipative (ESD) protection combined with low outgassing, extractable ionics and low particulation for clean room use Disk Drives Electrostatic dissipative (ESD) sheet and compounds for the packaging and transport of disk drive heads, internal disk drives and other sensitive components Electronic Assemblies Packaging Dissipative and conductive sheet for shipping and handling electronic sub-assemblies, filled circuit boards and other components that need reliable static charge dissipation Electronic Components Stat-Rite® polymer alloys meet the demanding specs for higher sensitivity to contaminants, particle generation and ESD as the space between circuitry and PCBs decreases Electronic Processing Products Specializing in permanent ESD protection to improve manufacturing yields and the reliability of devices and components after assembly is complete Industrial, Medical Package and Transport Semiconductors Tape Reel Shoes and Apparel.
Stat-Rite® Masterbatch Compounds - Conductive Polymers - The Lubrizol Corporation. Stat-Rite® Inherently Static Dissipative Masterbatch Compounds allow manufacturers the flexibility to incorporate static dissipative properties into their own materials and compounds, utilizing the cost saving benefits of combining application volumes on their base resin purchases.
Current Stat-Rite Polymer resins are manufactured as a complete compound and can be more expensive, whereas the masterbatch is produced as a concentrate that can be added to the customer's base resin. The Stat-Rite Masterbatches are availiable for the following base resins: • HIPS• ABS• Acrylic• Polyethylene• Polypropylene• PVC As one of the world leaders in ESD protection, more than anything, we provide the peace of mind - not only with products that consistently meet the highest quality standards, but also with one of the industry's largest network of technical support specialists.
When reliability, value and safety matter most, there is no better solution that one offered from Lubrizol Conductive Polymers. Polythiophene. The monomer repeat unit of unsubstituted polythiophene.
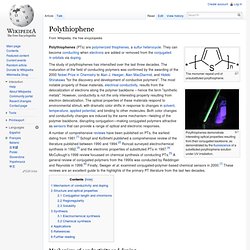
Polythiophenes demonstrate interesting optical properties resulting from their conjugated backbone, as demonstrated by the fluorescence of a substituted polythiophene solution under UV irradiation. The study of polythiophenes has intensified over the last three decades. The maturation of the field of conducting polymers was confirmed by the awarding of the 2000 Nobel Prize in Chemistry to Alan J. Heeger, Alan MacDiarmid, and Hideki Shirakawa "for the discovery and development of conductive polymers". The most notable property of these materials, electrical conductivity, results from the delocalization of electrons along the polymer backbone – hence the term "synthetic metals".
Mechanism of conductivity and doping[edit] Electrons are delocalized along the conjugated backbones of conducting polymers, usually through overlap of π-orbitals, resulting in an extended π-system with a filled valence band. Figure 1. Figure 2. Solubility[edit]