

Ammonia. LC50; Species: Campostoma anomalum (stoneroller); Concentration: 1.72 mg/L for 96 hr /Conditions of bioassay not specified/ European Commission, ESIS; IUCLID Dataset, Ammonia, anhydrous (CAS #7664-41-7) p.53 (2000 CD-ROM edition).
LC50; Species: Carassius auratus (goldfish); Concentration: 2-2.5 mg/L for 24-96 hr /Conditions of bioassay not specified/ Verschueren, K. Handbook of Environmental Data on Organic Chemicals. 3rd ed. New York, NY: Van Nostrand Reinhold Co., 1996., p. 186. BBC World Service - Elements, Nitrogen (N) - explosives. BBC World Service - Elements, Nitrogen (N) - fertilisers. Ammonia. The manufacture of ammonia is crucial for the world's agricultural industry for from it all fertilizers that contain nitrogen are produced.
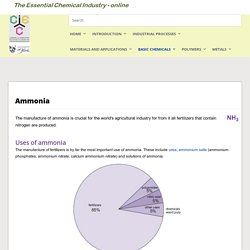
Uses of ammonia The manufacture of fertilizers is by far the most important use of ammonia. These include urea, ammonium salts (ammonium phosphates, ammonium nitrate, calcium ammonium nitrate) and solutions of ammonia. Figure 1 The uses of ammonia. An increasing amount of ammonia, although still small compared with other uses, is used as a concentrated solution in combating the discharge of nitrogen oxides from power stations. Annual production of ammonia Ammonia ranks second, to sulfuric acid, as the chemical with the largest tonnage.
Data from:U.S. Ammonia. Alchemy: Ammonia- Learn Chemistry. Follow the link and then select "Ammonia" from the left hand navigation menu.
Credits and acknowledgements The Royal Society of Chemistry thanks the following individuals and organisations for their help in producing the original video clips. Ammonia Uses and Benefits. What is ammonia used for?
About 90 percent of ammonia produced is used in fertilizer, to help sustain food production for billions of people around the world. Ammonia has other important uses; for example in household cleaning products and in manufacturing other products. What is ammonia? Ammonia, also known as NH3, is a colorless gas with a distinct odor composed of nitrogen and hydrogen atoms. It is produced naturally in the human body and in nature—in water, soil and air, even in tiny bacteria molecules. What happens to ammonia in the environment? BBC Bitesize - GCSE Chemistry (Single Science) - Ammonia and sulfuric acid - Revision 1. Ammonium nitrate and explosives. Ammonium nitrate fuel oil (ANFO) explosives are manufactured easily by combining the correct proportions of explosively reactive substances.
Commercial explosives are commonly available in the mining and farming industries in Western Australia and could be diverted for terrorist and criminal purposes. The regulation of what is generally termed as Security-Sensitive Ammonium Nitrate was considered by the Council of Australian Governments (COAG) on 25 June 2004 and similar licensing regimes were implemented by states and territories. What Causes Fertilizer Explosions. A fire anywhere is cause for concern, but a fire at a fertilizer plant is a potential catastrophe.
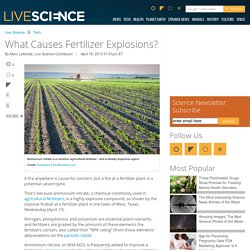
That's because ammonium nitrate, a chemical commonly used in agricultural fertilizers, is a highly explosive compound, as shown by the massive fireball at a fertilizer plant in the town of West, Texas, Wednesday (April 17). Nitrogen, phosphorous and potassium are essential plant nutrients, and fertilizers are graded by the amounts of these elements the fertilizers contain, also called their "NPK rating" (from those elements' abbreviations on the periodic table). Ammonium nitrate, or NH4-NO3, is frequently added to improve a fertilizer's nitrogen content. It's relatively stable under most conditions and is inexpensive to manufacture, according to Slate, making the chemical a popular alternative to other, more expensive nitrogen sources. But ammonium nitrate has a potentially lethal downside: If it comes into contact with an open flame or other ignition source, it explodes violently. Chemical Manufacturers Australia.
Ammonium hydroxide. LC50 Lepomis macrochirus (bluegill) 0.024-0.093 mg/l/48 hr.
/Conditions of bioassay not specified/ USEPA; Ambient Water Quality Criteria Doc: Ammonia p.151 (1984) EPA 440/5-85-001 LC50 Ictalurus punctatus (channel catfish) 0.974 mg/liter/one week pH= 7.7 Temp= 21.1 degrees C. Questions and Answers about Ammonium Hydroxide Use in Food Production - IFIC Foundation - Your Nutrition and Food Safety Resource. CSIRO – Ammonia Energy. Where will fuel cell vehicles (FCVs) first achieve critical mass?
Japan and California spring to mind as likely jurisdictions. South Korea not so much. That situation could change, though, with recent announcements from the Ministry of Trade, Industry, and Energy (MTIE) in Seoul. In fact, planned public and private sector investments could push South Korea to the front of the FCV pack. But while hydrogen-related activity of this nature can create opportunities for ammonia energy, the question always looms: are the key players in the implementing jurisdiction aware of the enabling roles ammonia can play?
Read more ... On July 13, Science magazine, the flagship publication of the American Association for the Advancement of Science (AAAS), published a 2,800-word “feature article" on ammonia energy. MacFarlane helped launch the Australian chapter of the NH3 Fuel Association. Read more ... Commonwealth Scientific and Industrial Research Organisation, Australian Government. ScienceDirect Topics.