

BU-310: How does Cobalt Work in Li-ion? Battery University BU-310: How does Cobalt Work in Li-ion?
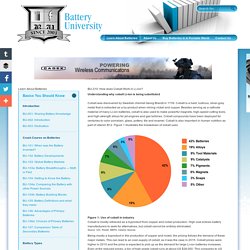
Understanding why cobalt Li-ion is being substituted Cobalt was discovered by Swedish chemist Georg Brandt in 1739. Cobalt is a hard, lustrous, silver-gray metal that is extracted as a by-product when mining nickel and copper. Besides serving as a cathode material of many Li-ion batteries, cobalt is also used to make powerful magnets, high-speed cutting tools, and high-strength alloys for jet engines and gas turbines.
Cobalt compounds have been deployed for centuries to color porcelain, glass, pottery, tile and enamel. Figure 1: Use of cobalt in industry Cobalt is mostly retrieved as a byproduct from copper and nickel production. Being mostly a byproduct in the production of copper and nickel, the pricing follows the demand of these major metals. According to the British Geological Survey (2014), the Democratic Republic of Congo has a 50 percent share of worldwide cobalt production. BU-309: How does Graphite Work in Li-ion? Understanding the key raw materials of Li-ion In 2015, the media predicted heavy demand for graphite to satisfy the growth of Li-ion batteries used in electric vehicles.

Speculation arose that graphite could be in short supply because a large EV battery requires about 25kg (55lb) of graphite for the anode. Although the price and consumption has been lackluster, there are indications that the demand might tighten. Producing anode-grade graphite with 99.99 percent purity is expensive and the process creates waste. The end-cost is not so much the material but the purification process. Carbon and graphite are related substances. Graphite comes from the Greek word “graphein.” Availability of Lithium. Battery University BU-308: Availability of Lithium Discover what is hype and reality, and what counts most.
The demand for Li-ion batteries is increasing and finding a sufficient supply of lithium as a raw material is kindling the mining industry. A compact EV battery (Nissan Leaf) uses about 4kg (9 lb) of lithium. If every man, woman and teenager were to drive an electric car in the future, a lithium shortage could develop and rumor of this happening has been spreading, perhaps prematurely. Most of the known supply of lithium is in Bolivia, Argentina, Chile, Australia and China. *** Please Read Regarding Comments *** Comments are intended for "commenting," an open discussion amongst site visitors.
If you have a question, require further information, have a suggestion or would like to report an error, use the "contact us" form or email us at: answers@cadex.com. Previous Lesson Next Lesson Or Jump To A Different Article Basics You Should Know The Battery and You Batteries as Power Source J. Regulations on Building a Lithium-ion Pack. Examine the requirements for agency approval when building a Li-ion pack.
Building a Li-ion battery pack begins by filling voltage and runtime requirements, and then taking into account loading, environmental and footprint conditions. Portable designs for consumer products often ask for a slim profile and the most common choice is a prismatic or pouch cell. If space allows, the 18650 provides lower cost and generally better performance that the prismatic/pouch cell in terms of specific energy and durability. The 18650 was originally designed for laptops, but with the profile of consumer products getting slimmer, research is shifting to the narrow cell formats.
BU-304b: Making Lithium-ion Safe. Battery packs using Li-ion require a mandatory protection circuit to assure safety under (almost) all circumstances.
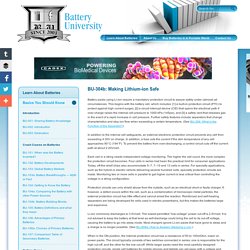
This begins with the battery cell, which includes: [1] a built-in protection circuit (PTC) to protect against high current surges, [2] a circuit interrupt device (CID) that opens the electrical path if over-charge raises the internal cell pressure to 1000 kPa (145psi), and [3] a safety vent that releases gas in the event of a rapid increase in cell pressure. Further safety features include separators that change characteristics and stop ion flow when exceeding a certain temperature.
(See BU-306: What is the Function of the Separator?) In addition to the internal cell safeguards, an external electronic protection circuit prevents any cell from exceeding 4.30V on charge. In addition, a fuse cuts the current if the skin temperature of any cell approaches 90°C (194°F). Each cell in a string needs independent voltage monitoring. Safety Concerns with Li-ion Batteries. Learn what causes Li-ion to fail and what to do in case of fire.

Safety of lithium-based batteries has attracted much media and legal attention. Any energy storage device carries a risk and this already occurred in the 1800s when steam engines exploded and people got hurt. Carrying highly flammable gasoline in cars was a hot topic in the early 1900s. Battery makers are obligated to meet safety requirements, but there are shortcuts by less reputable firms and it’s “buyer beware!”
Lithium-ion is safe but with millions of consumers using batteries, failures are bound to happen. Battery manufacturers strive to minimize the presence of such particles; however, complex assembly techniques make the elimination of all metallic dust a challenge. Li-ion using conventional metal oxides is nearing its theoretical limit on specific energy. There are two basic types of battery failures. Let’s examine the inner workings of the cell closer. Uneven separators can also trigger cell failure.