

TU Delft: Solid rocket propellants and their properties. Experimental investigation of the factors affecting the burning rate of solid rocket propellants. Modeling of combustion and ignition of solid-propellant ingredients. Abstract Techniques for modeling energetic-material combustion and ignition have evolved tremendously in the last two decades and have been successfully applied to various solid-propellant ingredients.
There has been a paradigm shift in the predictive capability of solid-propellant combustion models as the field has advanced from a simple and global-kinetics approach to a detailed approach that employs elementary reaction mechanisms in the gas phase, and accommodates thermal decomposition and subsequent reactions in the condensed phase. The detailed models not only allow calculation of propellant burning-rate characteristics, such as pressure and temperature sensitivities, but also of the surface conditions and entire combustion-wave structure, including the spatial variations in temperature and species concentrations. Home Made Explosives, Improvised Pyrotechnics, Rockets & Fireworks. Phantom Fireworks : History of Fireworks. Fireworks originated in China some 2,000 years ago.
The most prevalent legend has it that fireworks were discovered or invented by accident by a Chinese cook working in a field kitchen who happened to mix charcoal, sulphur and saltpeter (all commonly found in the kitchen in those days). The mixture burned and when compressed in an enclosure (a bamboo tube), the mixture exploded. Some sources say that the discovery of fireworks occurred about 2,000 years ago, and other sources place the discovery sometime during the 9th century during the Song dynasty (960-1279), although this could be confusion between the discovery of gunpowder by the cook and the invention of the firecracker.
Some sources suggest that fireworks may have originated in India, but in the October 18, 2003, online edition of The Hindu, an Indian national newspaper, the Chinese are credited with the discovery of gunpowder. The English were also fascinated with fireworks. The B.J. Ballistite. Ballistite is a smokeless propellant made from two high explosives, nitrocellulose and nitroglycerine.
It was developed and patented by Alfred Nobel in the late 19th century. The development of smokeless powders[edit] Cordite. A stick of cordite from World War II.
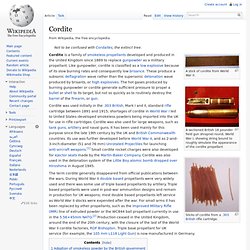
A sectioned British 18 pounder field gun shrapnel round, World War I, showing string bound to roughly simulate the appearance of the cordite propellant Cordite was used initially in the .303 British, Mark I and II, standard rifle cartridge between 1891 and 1915; shortages of cordite in World War I led to United States–developed smokeless powders being imported into the UK for use in rifle cartridges. Cordite was also used for large weapons, such as tank guns, artillery and naval guns. It has been used mainly for this purpose since the late 19th century by the UK and British Commonwealth countries.
Its use was further developed before World War II, and as 2-and-3-inch-diameter (51 and 76 mm) Unrotated Projectiles for launching anti-aircraft weapons.[1] Small cordite rocket charges were also developed for ejector seats made by the Martin-Baker Company. Adoption of smokeless powder by the British government[edit] Early European smokeless powders[edit] Sharing some information and experience on Ammonium perchlorate, Potassium nitrate-sucrose, and Zinc Sulfur based propellants... Sharing some information and experience on Ammonium perchlorate, Potassium nitrate-sucrose, and Zinc Sulfur based propellants.
Sharing some information/ experience on Ammonium perchlorate based propellants, Potassium nitrate sucrose propellants, and Zinc Sulfur propellants. Ammonium perchlorate based propellants(APCP) introduction In APCP, you'll need to use Ammonium perchlorate spherical so it allows better burning characteristics and easier mixing(casting). You can buy them from chemical stores that sells it for pyrotechnique purposes. Energic mixtures based on lead dioxide. Finely powdered Lead dioxide and fine Aluminum powder makes a flashpowder fast thermite, as does black Copper oxide.
The mix is static, shock and friction sensitive, I would not care to handle it in any quantity. As noted, you really don't want to breath the smoke. Your Lead is contaminated with Calcium, Bismuth and ARSENIC if it came from a modern storage battery. Nasty stuff, not a good choice for a starting material. Sell used batteries to a scrap yard and use the money to buy cleaner materials.
Sparkler Bombs. The friendly face of amateur pyrotechnics.
Don't believe me? Click here! Barium nitrate from sparklers. Might want to look at the toxicity of water soluble Barium compounds before you go about the extraction, and plan carefully to avoid contact with solutions, dust or fumes.
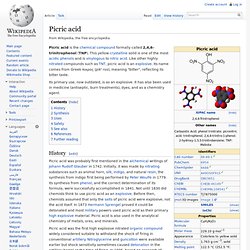
Barium carbonate (reacts to form soluble Barium chloride in the stomach) was a popular RAT POISON before the modern anticoagulant poisons were invented. Hgh Hydrogen content explosive compounds. Picric acid. Picric acid is the chemical compound formally called 2,4,6-trinitrophenol (TNP).
How to Make Fireworks. Homemade Fireworks Projects - Fun Fireworks Projects to Make Fireworks Yourself. Have you ever wanted to make your own fireworks?
These are instructions for fireworks projects so you can make your own homemade fireworks. Make Homemade Firecrackers Jeff Harris Photography / Getty Images It's easy and inexpensive to make firecrackers yourself. How to Make Slow Burning Fuses from Yarn, Sugar, and Potassium Nitrate. Here's how to make a simple form of a slow burning fuse from materials around the house. WARNING: Ignition of an incendiary or explosive material may not be legal in your area, so check local laws before attempting. Use of this video content is at your own risk. I made these fuses out of 100% cotton yarn, soaked in a solution of KNO3 (potassium nitrate) and white table sugar. Mix together a composition of 36 grams KNO3, and 24 grams of white table sugar and shake them together to make sure they are well mixed.
Next, boil 1/3 cup of water and stir in the composition until it's completely dissolved. Measure out about 12 feet of 100% cotton yarn, and soak it in the solution, and at any point now the yarn can be removed and strung out on a cookie sheet in a zigzag pattern. Preheat your oven to 300ºF and bake for 20 minutes. Then let cool for 5-10 minutes. Citric acid and its products. Dimethyl ether. Dimethyl ether (DME), also known as methoxymethane, is the organic compound with the formula CH 3OCH 3. The simplest ether, it is a colourless gas that is a useful precursor to other organic compounds and an aerosol propellant. Production[edit] Hydrazine. Molecular structure and properties[edit] Hydrazine forms a monohydrate that is more dense (1.032 g/cm3) than the anhydrous material. Hydrazine can arise via coupling a pair of ammonia molecules by removal of one hydrogen per molecule.
Acetic anhydride. Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula (CH3CO)2O.