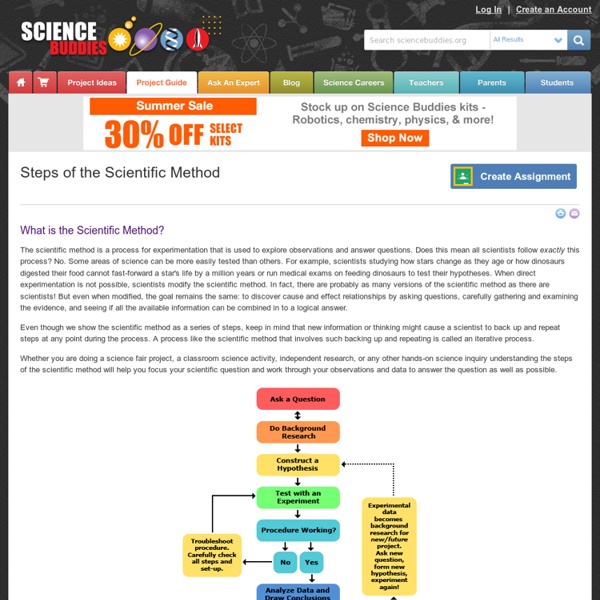
Steps of the Scientific Method
Please ensure you have JavaScript enabled in your browser. If you leave JavaScript disabled, you will only access a portion of the content we are providing. <a href="/science-fair-projects/javascript_help.php">Here's how.</a> What is the Scientific Method? The scientific method is a process for experimentation that is used to explore observations and answer questions. Even though we show the scientific method as a series of steps, keep in mind that new information or thinking might cause a scientist to back up and repeat steps at any point during the process. Whether you are doing a science fair project, a classroom science activity, independent research, or any other hands-on science inquiry understanding the steps of the scientific method will help you focus your scientific question and work through your observations and data to answer the question as well as possible. Educator Tools for Teaching the Scientific Method
Introduction to the Scientific Method
Introduction to the Scientific Method The scientific method is the process by which scientists, collectively and over time, endeavor to construct an accurate (that is, reliable, consistent and non-arbitrary) representation of the world. Recognizing that personal and cultural beliefs influence both our perceptions and our interpretations of natural phenomena, we aim through the use of standard procedures and criteria to minimize those influences when developing a theory. As a famous scientist once said, "Smart people (like smart lawyers) can come up with very good explanations for mistaken points of view." I. 1. 2. 3. 4. If the experiments bear out the hypothesis it may come to be regarded as a theory or law of nature (more on the concepts of hypothesis, model, theory and law below). II. As just stated, experimental tests may lead either to the confirmation of the hypothesis, or to the ruling out of the hypothesis. Error in experiments have several sources. III. IV. V. VI. VII. 1. 2. 3.
Interactive Practice on Physical vs. Chemical Properties
Review of Chemical vs. Physical Properties and Changes Chemical vs. Chemical properties are properties of an element or compound in chemical reactions. Physical properties are properties of an element or compound that can be observed without a chemical reaction of the substance. Chemical vs. In a physical change , the substances are not altered chemically, but merely changed to another phase (i.e. gas, liquid, solid) or separated or combined. In a chemical change , the substances are altered chemically and display different physical and chemical properties after the change. Practice on Identifying Chemical and Physical Properties Water boils at 100 degrees Celcius. Diamonds are capable of cutting glass. Practice on Identifying Chemical and Physical Changes Dry ice, solid carbon dioxide, is sublimed at room temperature. Salt is dissolved in water. Identifying Different types of Matter.
Chemical & Physical Changes
Chemical and physical changes are related to chemical and physical properties. Chemical Changes Chemical changes take place on the molecular level. A chemical change produces a new substance. Examples of chemical changes include combustion (burning), cooking an egg, rusting of an iron pan, and mixing hydrochloric acid and sodium hydroxide to make salt and water. Physical Changes Physical changes are concerned with energy and states of matter. How to Tell Chemical & Physical Changes Apart A chemical change makes a substance that wasn't there before. More Examples of Chemical and Physical ChangesPhysical and Chemical Changes QuizList of 10 Physical ChangesList of 10 Chemical Changes Stay up to date on the latest chemistry news, learn chemistry, and get instructions for chemistry projects.
Related:
Related: