Biofuel
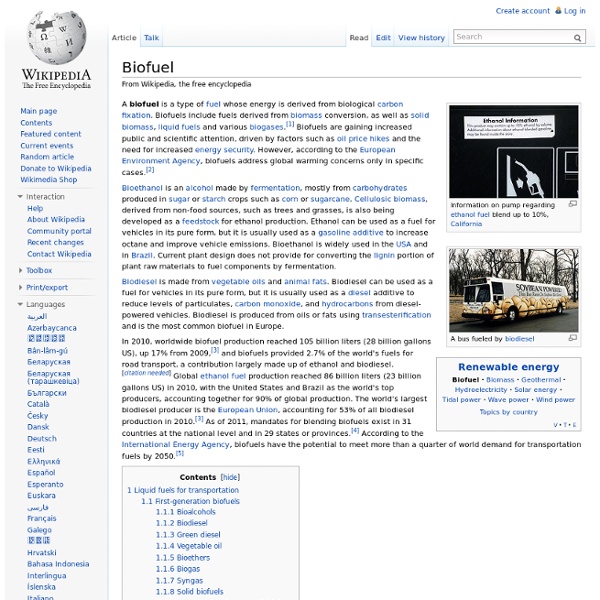
How Biomass Energy Works
Biomass is a combination of organic and biological materials usually composed of both plants and animals. It is composed of stored energy derived from the sun, and can release a new source of energy when physical and chemical processes are applied to it. Most biomasses materials come from wood, trees, grasses, agricultural crops, garbage and other urban wastes. • The most well known source of biomass energy is by burning of wood. • There is another simple way of how biomass energy works. • Agricultural crops like corn and sugarcane undergo the fermentation process to produce fuel like ethanol, which is used for trucks, cars and other means of transport. • Vegetable oils and animal fats can be converted to produce biodiesel which is also used in vehicles and other kinds of transportation. • Biomass energy also works by anaerobic digestion which involves the process of using microorganisms to break down biodegradable wastes and convert them into fuel.
Advantages and Disadvantages of Biomass
Speaking of resources, the world changes pace everyday. First there was evolution which took us from wood to steel to iron (and more), then development happened and took us to the stage that we are in now. Now we see that there are distinct shifts in policies which have taken us back to our roots and have been able to reacquaint us with the natural resources that we once used. One of those resources was biomass. This article will try to give you an in-depth review on the advantages and disadvantages of biomass energy. What is Biomass Biomass is biological matter that is acquired from plants and animals and has large amounts of carbon as well as traces of oxygen, nitrogen, hydrogen, alkaline earth and heavy metals in it. The main sources that are used to formulate biomass are wood, alcohol fuels and solid waste. Advantages of Biomass Uses Renewable Resources: Biomass is derived from sources like plants and animals, in short, sources which are replaceable. Disadvantages of Biomass
Biomass energy pros and cons in a nutshell | Renewable Green Energy Power
Biomass energy is a renewable energy source derived from biological materials that are called biomass. As with all renewable energy sources biomass energy has its pros and cons. Biomass Fuels The most common sources of biomass are wood, waste of biological form (i.e. sawdust), animal manure, landfill gasses, biofuel crops (i.e corn) and garbage (Municipal Solid Waste). In effect biomass energy results from sun. Biomass energy is one of the first forms of energy used by humans. Picture 1 Biomass Energy Pros Renewable energy. Biomass Energy Cons Harmful emissions Even though biomass energy production is carbon neutral it involves emission of other gases that can be harmuful to the environment. Biomass energy is renewable and can be sustainable if we use it wisely. About the Author Peter is a data analyst with over a decade of experience in environmental data analysis. Contact the author
Related:
Related:



