MDMA / Ecstasy : Utopian Pharmacology
Senegalia berlandieri
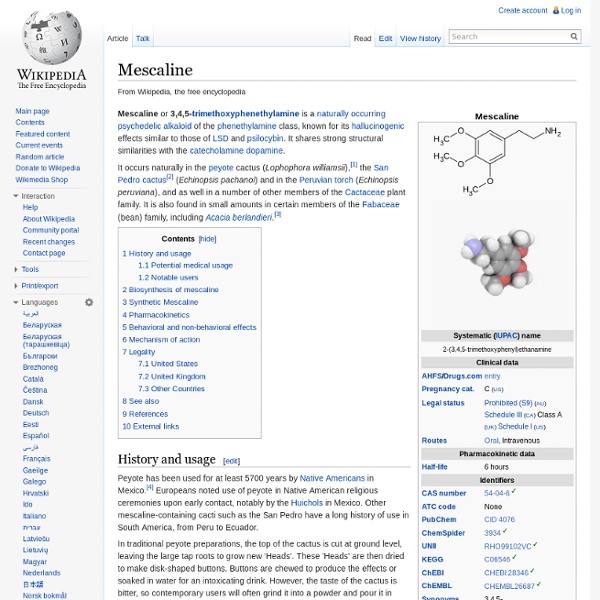
Species of plant Senegalia berlandieri (Berlandier acacia, guajillo acacia, guajillo, huajillo, huajilla) is a shrub native to the Southwestern United States and northeast Mexico that belongs to the Mimosoid clade of Fabaceae. It grows 1 to 5 metres (3.3 to 16.4 ft) tall, with blossoms that are spherical and white, occurring from February through April.[1] The berlandieri epithet comes from the name of Jean-Louis Berlandier,[2] a French naturalist who studied wildlife native to Texas and Mexico. S. berlandieri contains a wide variety of alkaloids and has been known to cause toxic reactions in domestic animals such as goats.[3][4] Uses[edit] Senegalia berlandieri is toxic to livestock and thus should not be used as forage or fodder.[5] Alkaloids[edit] Illicit use in supplements[edit] Gallery[edit] Senegalia berlandieri foliage and flowers Senegalia berlandieri tree Senegalia berlandieri bark Senegalia berlandieri flowers and seed pods Senegalia berlandieri seeds References[edit]
Dimethyltryptamine
History[edit] Another historical milestone is the discovery of DMT in plants frequently used by Amazonian natives as additive to the vine Banisteriopsis caapi to make ayahuasca decoctions. Biosynthesis[edit] Biosynthetic pathway for N,N-dimethyltryptamine This transmethylation mechanism has been repeatedly and consistently proven by radiolabeling of SAM methyl group with carbon-14 (14C-CH3)SAM).[22][20][24][25][26] Evidence in mammals[edit] In 2013, researchers first reported DMT in the pineal gland microdialysate of rodents.[28] A study published in 2014 reported the biosynthesis of N,N-dimethyltryptamine (DMT) in the human melanoma cell line SK-Mel-147 including details on its metabolism by peroxidases. [29] In a 2014 paper, a group first demonstrated the immunomodulatory potential of DMT and 5-MeO-DMT through the Sigma-1_receptor of human immune cells. INMT[edit] Endogenous DMT[edit] The first claimed detection of mammalian endogenous DMT was published in June 1965: German researchers F.
Bruno Schulz
Polish Jewish writer and artist Biography[edit] Schulz was born in Drohobych, Austrian Galicia, historically part of the Kingdom of Poland before the three partitions, and today part of Ukraine. Schulz was discouraged by influential colleagues from publishing his first short stories. Writings[edit] Schulz's body of written work is small; The Street of Crocodiles, Sanatorium Under the Sign of the Hourglass and a few other compositions that the author did not add to the first edition of his short story collection. Both books were featured in Penguin's series "Writers from the Other Europe" from the 1970s. Madeline G. In 2020, Sublunary Editions published Frank Garrett's translation of Undula, an early story by Schulz which appeared in Dawn: The Journal of Petroleum Officials in Boryslav under the pseudonym Marceli Weron.[12][13] Adaptations[edit] Schulz's work has provided the basis for two films. Literary references and biography[edit] Mural controversy[edit] Notes[edit] References[edit]
The Informed Pagan | promoting responsibility and integrity within the pagan community, through knowledge and awareness.
Nicotinamide adenine dinucleotide
Chemical compound which is reduced and oxidized Chemical compound In organisms, NAD can be synthesized from simple building-blocks (de novo) from either tryptophan or aspartic acid, each a case of an amino acid. Alternatively, more complex components of the coenzymes are taken up from nutritive compounds such as niacin; similar compounds are produced by reactions that break down the structure of NAD, providing a salvage pathway that recycles them back into their respective active form. Some NAD is converted into the coenzyme nicotinamide adenine dinucleotide phosphate (NADP), whose chemistry largely parallels that of NAD, though its predominant role is as a coenzyme in anabolic metabolism. In the name NAD+, the superscripted plus sign indicates the positive formal charge on one of its nitrogen atoms. Physical and chemical properties[edit] Nicotinamide adenine dinucleotide consists of two nucleosides joined by pyrophosphate. NAD+ and NADH also differ in their fluorescence. Biosynthesis[edit]
Polymath
Individual whose knowledge spans a substantial number of subjects A polymath (Greek: πολυμαθής, polymathēs, "having learned much"; Latin: homo universalis, "universal person")[1] is an individual whose knowledge spans a substantial number of subjects, known to draw on complex bodies of knowledge to solve specific problems. In Western Europe, the first work to use the term polymathy in its title (De Polymathia tractatio: integri operis de studiis veterum) was published in 1603 by Johann von Wowern, a Hamburg philosopher.[2][3][4] Von Wowern defined polymathy as "knowledge of various matters, drawn from all kinds of studies ... ranging freely through all the fields of the disciplines, as far as the human mind, with unwearied industry, is able to pursue them".[2] Von Wowern lists erudition, literature, philology, philomathy and polyhistory as synonyms. Renaissance man[edit] In academia[edit] Robert Root-Bernstein and colleagues[edit] Peter Burke[edit] Kaufman, Beghetto and colleagues[edit]
1. How different weighting methods work - Pew Research Center Methods
Historically, public opinion surveys have relied on the ability to adjust their datasets using a core set of demographics – sex, age, race and ethnicity, educational attainment, and geographic region – to correct any imbalances between the survey sample and the population. These are all variables that are correlated with a broad range of attitudes and behaviors of interest to survey researchers. Additionally, they are well measured on large, high-quality government surveys such as the American Community Survey (ACS), conducted by the U.S. Census Bureau, which means that reliable population benchmarks are readily available. But are they sufficient for reducing selection bias in online opt-in surveys? Two studies that compared weighted and unweighted estimates from online opt-in samples found that in many instances, demographic weighting only minimally reduced bias, and in some cases actually made bias worse. Raking Matching We used this similarity measure as the basis for matching.