

Acid-base titrations 2: the acid content of individual sour hard candies. Chemdemos. College of Science. Chemistry Teacher International Volume 1 Issue 2 (2019) Introducing spectrophotometry in the school lab employing LEGO bricks and LEDs in: Chemistry Teacher International Volume 1 Issue 1 (2019) Where to Find IB Chemistry Past Papers - Free and Official. Taking the IB Chemistry exam will be a nerve-wracking experience, but having seen a real IB past paper before taking the actual test will be a huge advantage since you'll have experience with the test format, the length, and style of the test.
In this article, I will show you where to find IB Chemistry past papers, both free and paid. I'll also share tips on how to study most effectively using these IB Chemistry past papers. Where to Find Free Tests The IB has been diligent about seeking out and destroying illegally uploaded official papers for the past few years, so a lot of sources that used to be out there are no longer readily available. Balancing redox equations - Practice exercises. Ch 13 - NMR basics. SN1 & SN2 Quiz. Artificial vs Natural Peach. This artificial vs natural foods phenomenon has grown somewhat since the All-Natural Banana.
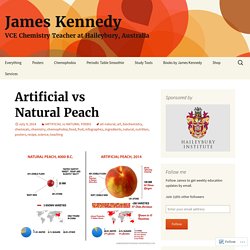
This infographic explores the differences between the natural, “wild peach” and its modern, artificial relative. It explores how the ancient Chinese developed a small, wild fruit (that tasted like a lentil) into the juicy, delicious peaches that we eat today. This image also pays homage to the thousands of years of toil that farmers put into developing the Peach regardless of whether they were aware of it consciously or not. After the wild peach was domesticated in 4000 B.C., farmers selected seeds from the tastiest fruits for re-planting.
They tended to the trees for thousands of years, and the fruits became bigger and juicier with each generation. Which one would you rather eat? 24.4: The Nernst Equation - Chemistry LibreTexts. The "Invisible Reality" of Quantum Theory with Alan Alda, Brian Greene, and others. IB Chemistry IA and EE - MrWeng's IB Chemistry. How to get a 7 in your Chemistry IA IB Chemistry IA Exemplar - this is what you are aiming for...

Resources: THE SECRET WEAPON: Internal Assessment (and foundation for Extended Essay) Science Report Template: Title: Must include the dependent and independent variable, must be specific. IntroductionStart with a few sentences on your personal interest in this investigation. Materials Use the smallest quantity measured, and so always use the largest uncertainties for your uncertainty propagation calculations. Qualitative Observations:You must give qualitative observations to supplement the quantitative data. Redox 5 hydrogen electrode animation. Home. CLP quiz - ECHA. GHS quiz: Match the pictogram to the hazard. CUP IBChemistry c06 it. Vklass learning platform - Login. Cambridge Chemistry Challenge : C3L6. IB Biology/Chemistry: Error/Uncertainty. IB Chemistry,Uncertainty, Error Analysis, Standard Deviation Uncertainty Calculation for Rate and Concentration of reaction.

Rate = 1/time, Time for X to disappear. ( Iodine Clock Reaction) 3 Methods for Uncertainty Calculation for Rate (0.10M) KI. Average time is (5.28 + 4.75 + 4.47) / 3 = 4.83. CORE_Chapter Fourteen - Gas Phase, Solubility, Complex Ion Equilibria. NO2N2O4 Equilibrium This animation shows the effect of change the volume of a gas phase equilibrium mixture where the numbers of reactant and product molecules are different.

CaO CaCO3 Equilibrium This animation shows a solid and gas equilibrium system and the effect adding additional solid on the position of the equilibrium. Energy of Activation This animation shows the change in the number of molecules with energy greater than the energy of activation as the temperature increases. H2 I2 Equilibrium This animation shows the effect of change the volume of a gas phase equilibrium mixture where the numbers of reactant and product molecules are the same. N2 O2 Equilibrium This animation shows a gas phase equilibrium system. Course: Grade 12 IB Chemistry. Getting your practical programme together - OSC IB Blogs. Hopefully, by now you will have had time to reflect on the new course and start thinking about your practical programme (PSOW).

It is my intention of this blog post ot give you some ideas of things you can add to your PSOW. Are you aware of the ten mandatory labs / skills that you are expected to teach your students? These are listed in the guide ….. but they are not very clear. If you look at the biology guide you will see that they are signposted ‘experiment 1’, ‘experiment 2’, etc – but not in chemistry. Gel Electrophoresis. IB Chemistry revision notes and syllabus.
Molecule Shapes 1.1.8 7.6 Molecular Structure and Polarity. Learning Objectives By the end of this section, you will be able to: Predict the structures of small molecules using valence shell electron pair repulsion (VSEPR) theoryExplain the concepts of polar covalent bonds and molecular polarityAssess the polarity of a molecule based on its bonding and structure Thus far, we have used two-dimensional Lewis structures to represent molecules.
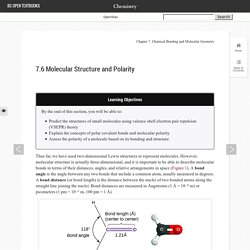
However, molecular structure is actually three-dimensional, and it is important to be able to describe molecular bonds in terms of their distances, angles, and relative arrangements in space (Figure 1). A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees. Valence shell electron-pair repulsion theory (VSEPR theory) enables us to predict the molecular structure, including approximate bond angles around a central atom, of a molecule from an examination of the number of bonds and lone electron pairs in its Lewis structure. Infographics. Biochemical-pathways. 3-D Biological Molecules. Listed below are links to pages containing 3-dimensional displays of models of molecules of Biological interest.

These may be moved in an intuitive way using the computer mouse or touchscreen. In the explanatory text are links which highlight features of the molecule or give extra information. These units are now based on HTML5 and javascript, so they should be more accessible from most PCs and tablets, including iPads. These are mostly upgrades of my previous units on molecules which used applets based on Java. Mplex ions - colour. What about non-transition metal complex ions?

Non-transition metals don't have partly filled d orbitals. Visible light is only absorbed if some energy from the light is used to promote an electron over exactly the right energy gap. Non-transition metals don't have any electron transitions which can absorb wavelengths from visible light. For example, although scandium is a member of the d block, its ion (Sc3+) hasn't got any d electrons left to move around. This is no different from an ion based on Mg2+ or Al3+. Substitution in complex ions - ligand exchange. Replacing the water in the hexaaquacopper(II) ion This is a slightly untypical case, because only four of the six water molecules get replaced to give the tetraamminediaquacopper(II) ion, [Cu(NH3)4(H2O)2]2+.

Notice that the four ammonias all lie in one plane, with the water molecules above and below. What you see in a test tube is: Home › Eric Mazur. IB Chemistry: HL Questions on each sub-topic. Electron configuration. Online resources - Schools - Chemistry. What Is the Strong Force? The strong nuclear force is one of the four fundamental forces in nature; the other three are gravity, electromagnetism and the weak force. As its name implies, the strong force is the strongest force of the four. It is responsible for binding together the fundamental particles of matter to form larger particles. Acid-base reactions in solution. Rutherford Scattering - Quantum Mechanics, Atomic Nuclei, Rutherford Scattering.
Topics Atomic Nuclei Atomic Structure Quantum Mechanics Description How did Rutherford figure out the structure of the atom without being able to see it? Simulate the famous experiment in which he disproved the Plum Pudding model of the atom by observing alpha particles bouncing off atoms and determining that they must have a small core. Sample Learning Goals. IB Chemistry Internal Assessment. Guiding Question To what extent does the exploration have a clear and fully focused research question? A good research question is sharply focused and lends itself to an investigation that is sophisticated enough for IB level. The independent and dependent variables need to be clearly identified. In forming your question consider using an open ended command to help focus the question by directing it in a specific way.
IB Chemistry: Uncertainty & error questions. Chemistry teacher support material. Practical work is a vital and integral part of group 4 science courses, providing students with experience of investigative and experimental activities within and outside the classroom. It enables them to develop a wide range of skills such as investigation, design, manipulative skills, data processing and analysis, evaluation, teamwork and communication.
The opportunity to undertake investigations and hands-on experimentation allows them to engage in many of the processes encountered by scientists, and to appreciate the nature of scientific thought and investigation. Teachers will develop a practical scheme of work (PSOW) for each class that will be recorded on Form 4: PSOW. For an SL only class or an HL only class, only one 4/PSOW is required, but for a mixed SL/HL class, separate 4/PSOW forms are required for SL and HL. Prescribed practicals. IB Teacher Support Materials - Enhancing Chemistry Learning with ICT. Vocabulary List. Accelerate - to speed up Accelerator - a machine which accelerates charged particles to high energies Antimatter - matter that is exactly the opposite in every way from its matter counterpart: antiquark/quark; positron/electron Atom - the smallest unit of a chemical element, made up of a nucleus surrounded by electrons Beam - a ray of light; a group of particles traveling together along a well-defined path BEAMS - the acronym for Becoming Enthusiastic About Math and Science.
Redox 1 combining half cells. Order of reaction experiments. Some sample reactions. Exp16calcsW. NIST Chemical Kinetics Database. Free energy and equilibrium. 1 The road to equilibrium is down the free energy hill This means, of course, that if the total free energy G of a mixture of reactants and products goes through a minimum value as the composition changes, then all net change will cease— the reaction system will be in a state of chemical equilibrium . Spectroscopy in a Suitcase teacher's resource: UV / Visible Spectroscopy- Learn Chemistry.
A Level Period 4 Elements Survey trends in physical properties GCE AS A2 inorganic revision notes KS5. INORGANIC Part 6 Period 4 survey & group trends page sub–index: 6.1 Survey of Period 4 elements * 6.2 Period 4 element trends in physical properties * 6.3 Period 4 element trends in bonding and formulae * 6.4 Important element trends down a Group Advanced Level Inorganic Chemistry Periodic Table Index * Part 1 Periodic Table history * Part 2 Electron configurations, spectroscopy, hydrogen spectrum, ionisation energies * Part 3 Period 1 survey H to He * Part 4 Period 2 survey Li to Ne * Part 5 Period 3 survey Na to Ar * Part 6 Period 4 survey K to Kr and important trends down a group * Part 7 s–block Groups 1/2 Alkali Metals/Alkaline Earth Metals * Part 8 p–block Groups 3/13 to 0/18 * Part 9 Group 7/17 The Halogens * Part 10 3d block elements & Transition Metal Series * Part 11 Group & Series data & periodicity plots.
Kan du frågorna i Teknik-SM? Facit ProvI KO2016. Acid_base. Crm3s5 5. NRCFs frågelåda i fysik - nyckelord: absoluta nollpunkten. Fråga: Kan man ha temperaturer lägre än absoluta nollpunkten? The order of filling 3d and 4s orbitals. Chemistry calculations. There are relatively few calculations on this site, but you might be interested in my chemistry calculations book. If you have found this site helpful, you should find the book will help you as well. Classifying: Calm the Chaos. Grekisk filosof och tillsammans med Leukippos företrädare för den antika atomteorin. Gk3-6.pdf. A Brief Introduction to Mass Spectrometry. The Manhattan Project. The Moment in Time: The Manhattan Project.