

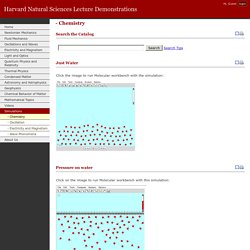
- Chemistry. Click the image to run Molecular workbench with the simulation: Click on the image to run Molecular workbench with this simulation: or the high pressure version:
Chemistry. Water Research. 19 Unique Properties of Water. Contact the Author in any language.

Why are you here? Were you able to complete your task? If not, why not? Section 14. Intermolecular Forces. Key Concepts Three types of force can operate between covalent molecules:

Intramolecular Forces: Chemical Bonds. Water and its structure. ↑[image] Note: this document will print in an appropriately modified format (14 pages) The molecule of water A molecule is an aggregation of atomic nuclei and electrons that is sufficiently stable to possess observable properties — and there are few molecules that are more stable and difficult to decompose than H2O.

In water, each hydrogen nucleus is bound to the central oxygen atom by a pair of electrons that are shared between them; chemists call this shared electron pair a covalent chemical bond. Water models. Water structure: Methods. Water absorption spectrum. Early Water Cluster Studies. Cooperativity in Large Water Clusters Liquid Water, Iceand Clathrates. John von Neumann Institute for Computing RogerA.

Klein publishedin NICSymposium2006, G.M unster,D.Wolf,M.Kremer(Editors), Johnvon Neumann Institute for Computing, Julich, NIC Series, Vol. 32,ISBN3-00-017351-X,pp. 65-74,2006. 2006 by Johnvon Neumann Institutefor Computing Permission to make digital or hardcopies of portions of this work for personal or classroomuseisgrantedprovidedthat thecopiesarenot madeor distributedfor pro t or commercial advantageandthat copies bear thisnoticeandthefull citationonthe rst page. Tocopyotherwise requirespriorspeci cpermission by the publishermentionedabove. www.fz-juelich.de/nic-series/volume32 Cooperativity in Large Water Clusters Liquid Water, Ice and Clathrates Roger A. Klein Institute for Physiological Chemistry University of Bonn, Nussallee 11, 53115 Bonn, Germany E-mail: klein@institut.physiochem.uni-bonn.de Кооперация больших водяных кластеров воды, льда и клатратов 1 Introduction Figure 1. 2 Background Figure 2. SURVEY-OF-I-CLUSTERS.
Spectroscopy. Vibrational spectra. Water. Atomic force microscopy water cluster analysis. Water cluster analysis. Seeing Double. © THOMAS DEERINCK, NCMIR/SCIENCE SOURCEFluorescent dyes and genetic tags have revolutionized researchers’ ability to determine the location of specific proteins in space and time using light microscopy (LM).

With the advent of super-resolution fluorescence LM techniques, much more precise location of proteins within a cell is possible, but those techniques do not provide information about the ultrastructural cellular context of biomolecules. Electron microscopy (EM) is an unbeatable tool for providing structural data at the nanometer scale, but the field of view in EM is very narrow and the molecular scenery is highly crowded, making it tedious and time-consuming to locate specific structures and cellular events, especially if they are rare.
Moreover, EM can only provide a static snapshot of the sample because cells and tissues must be fixed prior to imaging. Researchers have begun to develop techniques that help overcome these challenges. October 2005: Revealing the Mysteries of Water. Modern%20Structure%20Research%20of%20Clustered%20Water. The Chemistry of Water. Science@Berkeley Lab: Water: Dissolving the Controversy. You would think that if scientists could agree upon anything, it would be water.

After all, almost everyone knows that H2O is the symbol for water; two atoms of hydrogen attached to one atom of oxygen form a water molecule. What could be simpler than that? And yet chemists have been disagreeing for the past 40 years over how water molecules arrange themselves in a liquid drop. In this scientific controversy, one side has argued for what is called the two-state model.
The other side has argued for what is called the continuum model. This Is What Happens When You Blow Soap Bubbles at -9°C (15,8°F) Thanks! Like us on FB for more awesome posts! When the weather forecast announced about the unexpected cold from -9°C to -12°C last week, Washington-based photographer Angela Kelly decided to take an advantage of it in one truly creative way.
Together with her 7-year-old son, Kelly combined the home-based remedies – dish soap, karo syrup, and water – and went out to blow bubbles and take pictures as they freeze and melt. Soon the two adventurers found themselves in awe while watching the frost create magical patterns in the freezing bubbles. The smaller ones would freeze momentarily, simply mid-air, and then they would fall down and scatter like thin glass chips. “We noted how they would freeze completely before the sun rose but that once the sun was in view they would defrost along the tops or cease freezing altogether.“ recalls Kelly to the KOMO News. Photos: Bubbles crystallize into spherical beauty during freezing temps. What to do when you've got a creative mind, a knack for photography, and temperatures are hovering near single digits? Go blow bubbles! Then take pictures of what happens next. Arlington's Angela Kelly of Kelly Images and Photography has been featured in this blog before for her beautiful natural displays of dew drops and melting frost.
But when she heard we were about to be invaded by a chilly arctic wind, she decided to try her hand at something new: Frozen bubbles. "When I learned that we were expecting to see our coldest temperatures last week since last February, I knew I had to try to capture the ice we were bound to see it in its best (and most interesting) form," she told me. Photographer gets amazing pics of frozen bubbles and frost crystals. Arlington photographer Angela Kelly, who made waves around the Internet with her gallery of frozen bubbles featured in this blog during our last cold snap in December, was out again in the frigid mornings this week trying to add to her frozen bubble collection.
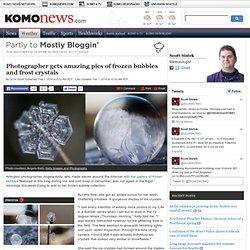
But this time, she got an added bonus for her teeth-chattering troubles: A gorgeous display of ice crystals. "I had every intention of adding more photos to my 'Life in a Bubble' series when I set out to work in the 12 degree temps (Thursday) morning," Kelly told me. "I was quickly distracted however by the glittering frost in the field. The field seemed to glow with twinkling lights and upon closer inspection, through the lens of my camera, I found that it was actually individual ice crystals that looked very similar to snowflakes. " She said the ice crystals had formed around the blades of grass and other debris due to the very light freezing fog they had overnight and the very low temperatures. Chemistry.