

Solid-State Battery. Zinc–carbon battery. Zinc–carbon batteries of various sizes.
Zinc–carbon batteries were the first commercial dry batteries, developed from the technology of the wet Leclanché cell (/lɛklɑːnˈʃeɪ/), and made flashlights and other portable devices possible, because the battery can function in any orientation. They are still useful in low drain or intermittent use devices such as remote controls, flashlights, clocks or transistor radios. Zinc–carbon dry cells are single-use primary cells, since they are not intended to be recharged. History[edit] Old 3V zinc–carbon battery, ca. 1960, with cardboard casing. Improvements include the use of purer grades of manganese dioxide, better sealing, and purer zinc for the negative electrode. Zinc–carbon battery. Zinc–carbon batteries of various sizes.
Zinc–carbon batteries were the first commercial dry batteries, developed from the technology of the wet Leclanché cell (/lɛklɑːnˈʃeɪ/), and made flashlights and other portable devices possible, because the battery can function in any orientation. They are still useful in low drain or intermittent use devices such as remote controls, flashlights, clocks or transistor radios. Zinc–carbon dry cells are single-use primary cells, since they are not intended to be recharged. History[edit] Old 3V zinc–carbon battery, ca. 1960, with cardboard casing. Improvements include the use of purer grades of manganese dioxide, better sealing, and purer zinc for the negative electrode.
Zinc–air battery. Zinc–air hearing aid batteries During discharge, a mass of zinc particles forms a porous anode, which is saturated with an electrolyte.
Oxygen from the air reacts at the cathode and forms hydroxyl ions which migrate into the zinc paste and form zincate (Zn(OH)2− 4), releasing electrons to travel to the cathode. The zincate decays into zinc oxide and water returns to the electrolyte. The water and hydroxyl from the anode are recycled at the cathode, so the water is not consumed. The reactions produce a theoretical 1.65 volts, but this is reduced to 1.35–1.4 V in available cells. Weston cell. Drawing from Edward Weston's US Patent 494827 depicting the standard cell.
Chemistry[edit] Water-activated battery. A water-activated battery is a disposable reserve battery that does not contain an electrolyte and hence produces no voltage until it is soaked in water for several minutes.
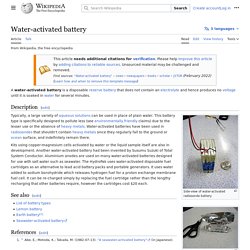
Description[edit] Side-view of water-activated radiosonde battery Radiosonde battery still in protective wrapper Typically, a large variety of aqueous solutions can be used in place of plain water.
Silver-oxide battery. A silver-oxide battery (IEC code: S) is a primary cell with a very high energy/weight ratio.
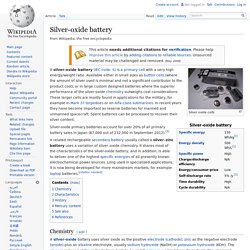
Available either in small sizes as button cells (where the amount of silver used is minimal and not a significant contributor to the product cost), or in large custom designed batteries where the superior performance of the silver-oxide chemistry outweighs cost considerations. These larger cells are mostly found in applications for the military, for example in Mark 37 torpedoes or on Alfa-class submarines.
In recent years they have become important as reserve batteries for manned and unmanned spacecraft. Reserve battery. A reserve battery, also called stand-by battery, is a primary battery where part is isolated until the battery needs to be used.
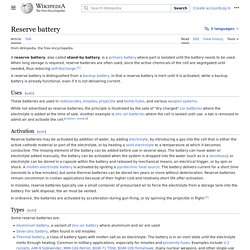
When long storage is required, reserve batteries are often used, since the active chemicals of the cell are segregated until needed, thus reducing self-discharge. [1] A reserve battery is distinguished from a backup battery, in that a reserve battery is inert until it is activated, while a backup battery is already functional, even if it is not delivering current. Uses[edit] These batteries are used in radiosondes, missiles, projectile and bomb fuzes, and various weapon systems. Pulvermacher's chain. Fig. 1.
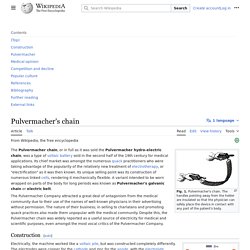
Pulvermacher's chain. The handles pointing away from the holder are insulated so that the physician can safely place the device in contact with any part of the patient's body. The Pulvermacher chain, or in full as it was sold the Pulvermacher hydro-electric chain, was a type of voltaic battery sold in the second half of the 19th century for medical applications. Its chief market was amongst the numerous quack practitioners who were taking advantage of the popularity of the relatively new treatment of electrotherapy, or "electrification" as it was then known.
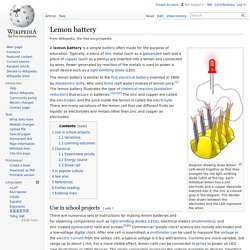
Its unique selling point was its construction of numerous linked cells, rendering it mechanically flexible. Lemon battery. Diagram showing three lemon cells wired together so that they energize the red light emitting diode (LED) at the top.
Paper battery. A paper battery is an ultra-thin electric battery engineered to use a spacer formed largely of cellulose (the major constituent of paper).
It incorporates nanoscale structures to act as high surface-area electrodes to improve the conduction of electricity.[1] Paper batteries are thin, flexible and environment-friendly, allowing integration into a wide range of products. Their functioning is similar to conventional chemical batteries with the important difference that they are non-corrosive and do not require a bulky housing. There are many uses for this new technology, including health care to power tiny medical diagnostic equipment and even drug delivery transdermal patches.
Organic radical battery. An organic radical battery (ORB) is a relatively new type of battery first developed in 2005.[1] This type of battery is generally not available for the consumer, however their development is approaching practical use.[2] ORBs are potentially more environmentally friendly than conventional metal-based batteries, because they use organic radical polymers, which are flexible plastics, instead of metals to provide electrical power. ORBs are considered to be a high-power alternative to the Li-ion battery. Functional prototypes of the battery have been researched and developed by different research groups and corporations including the Japanese corporation NEC.[1] Current ORB research is being directed mostly towards Hybrid ORB/Li-ion batteries because organic radical polymers with appropriate electrical properties for the anode are difficult to synthesize.[3] Applications[edit]
Mercury battery. Mercury battery "РЦ-53М", Russian manufactured in 1989 History[edit] The mercury oxide-zinc battery system was known more than 100 years ago[1] but did not become widely used until 1942, when Samuel Ruben developed a balanced mercury cell which was useful for military applications such as metal detectors, munitions, and walkie-talkies.[2] The battery system had the advantages of long shelf life (to 10 years) and steady voltage output. After the Second World War the battery system was widely applied for small electronic devices such as cardiac pacemakers and hearing aids.
Mercury oxide batteries were made in a range of sizes from miniature button cells used for hearing aids and electric wrist watches, cylindrical types used for portable electronic apparatus, rectangular batteries used for transistor radios,[3] and large multicell packs used for industrial applications such as radio remote control for overhead crane systems. Lithium–air battery. Originally proposed in the 1970s as a possible power source for battery electric vehicles, Li-air batteries recaptured scientific interest in the late 2000s due to advances in materials technology and an increasing demand for renewable energy sources.
The major appeal of the Li-air battery is the extremely high specific energy, a measure of the amount of energy a battery can store for a given weight. A lithium-air battery has an energy density (per kilogram) comparable to gasoline. Li-air batteries gain this advantage in specific energy since they use oxygen from the air instead of storing an oxidizer internally. The technology requires significant advances in multiple fields before a viable commercial implementation can be developed.[2] Four approaches are active: aprotic,[3][4] aqueous,[5] solid state,[6] and mixed aqueous/aprotic.[7] Metal-air batteries, specifically zinc-air, have received attention due to the potential for high energy densities. Lithium battery. This article is about disposable lithium batteries. It is not to be confused with Lithium-ion battery. Lithium 9 volt, AA, and AAA sizes.
The top unit has three lithium-manganese dioxide cells internally, the bottom two are lithium-iron disulfide single cells physically and electrically compatible with 1.5 volt zinc batteries. They stand apart from other batteries in their high charge density (long life) and high cost per unit. Depending on the design and chemical compounds used, lithium cells can produce voltages from 1.5 V (comparable to a zinc–carbon or alkaline battery) to about 3.7 V. Lithium batteries are widely used in products such as portable consumer electronic devices. Lemon battery. Diagram showing three lemon cells wired together so that they energize the red light emitting diode (LED) at the top. Leclanché cell.
Grove cell. The Grove cell was an early electric primary cell named after its inventor, British chemist William Robert Grove, and consisted of a zinc anode in dilute sulfuric acid and a platinum cathode in concentrated nitric acid, the two separated by a porous ceramic pot. Galvanic cell. A galvanic cell, or voltaic cell, named after Luigi Galvani, or Alessandro Volta respectively, is an electrochemical cell that derives electrical energy from spontaneous redox reactions taking place within the cell. Frog battery. Matteucci's frog battery, 1845 (top left); Aldini's frog battery, 1818 (bottom); apparatus for controlled exposure of gases to frog battery (top right). Earth battery. An Earth battery is a pair of electrodes made of two dissimilar metals, such as iron and copper, which are buried in the soil or immersed in the sea.
Earth batteries act as water activated batteries and if the plates are sufficiently far apart, they can tap telluric currents. Earth batteries are sometimes referred to as telluric power sources and telluric generators. Dry cell. Line art drawing of a dry cell: 1. brass cap, 2. plastic seal, 3. expansion space, 4. porous cardboard, 5. zinc can, 6. carbon rod, 7. chemical mixture. A dry cell uses a paste electrolyte, with only enough moisture to allow current to flow.
Unlike a wet cell, a dry cell can operate in any orientation without spilling, as it contains no free liquid, making it suitable for portable equipment. By comparison, the first wet cells were typically fragile glass containers with lead rods hanging from the open top and needed careful handling to avoid spillage. Daniell cell. The Daniell cell is also the historical basis for the contemporary definition of the volt, which is the unit of electromotive force in the International System of Units. The definitions of electrical units that were proposed at the 1881 International Conference of Electricians were designed so that the electromotive force of the Daniell cell would be about 1.0 volts.[1][2] With contemporary definitions, the standard potential of the Daniell cell at 25 °C is actually 1.10 V.[3] Clark cell. This article is about the electrochemical cell. For the polarographic DO measurement electrode, see clark electrode. Chromic acid cell. Bichromate cells. Left - single fluid, right - two fluid.
Bunsen cell.
Aluminium-ion battery. Aluminium–air battery. Alkaline battery.