

Amedeo Avogadro. Biography[edit] Amedeo Carlo Avogadro was born in Turin, Italy in 1776 to a noble family of Piedmont, Italy.
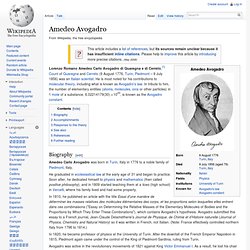
He graduated in ecclesiastical law at the early age of 31 and began to practice. Soon after, he dedicated himself to physics and mathematics (then called positive philosophy), and in 1809 started teaching them at a liceo (high school) in Vercelli, where his family lived and had some property. In 1810, he published an article with the title Essai d'une manière de déterminer les masses relatives des molécules élémentaires des corps, et les proportions selon lesquelles elles entrent dans ces combinaisons ("Essay on Determining the Relative Masses of the Elementary Molecules of Bodies and the Proportions by Which They Enter These Combinations"), which contains Avogadro's hypothesis.
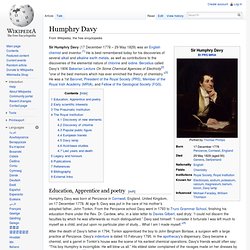
Humphry Davy. Sir Humphry Davy (17 December 1778 – 29 May 1829) was an English chemist and inventor.[1] He is best remembered today for his discoveries of several alkali and alkaline earth metals, as well as contributions to the discoveries of the elemental nature of chlorine and iodine.
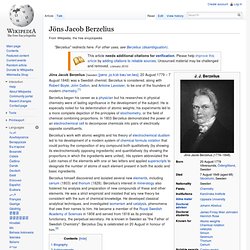
Berzelius called Davy's 1806 Bakerian Lecture On Some Chemical Agencies of Electricity[2] "one of the best memoirs which has ever enriched the theory of chemistry. Jöns Jacob Berzelius. Jöns Jacob Berzelius (Swedish: [jœns ˌjɑːkɔb bæɹˈseːliɵs]; 20 August 1779 – 7 August 1848) was a Swedish chemist.
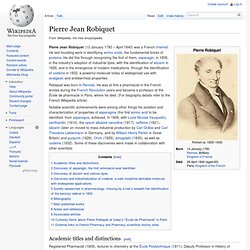
Pierre Jean Robiquet. Pierre Jean Robiquet (13 January 1780 – April 1840) was a French chemist.
He laid founding work in identifying amino acids, the fundamental bricks of proteins. Michael Faraday. Michael Faraday, FRS (22 September 1791 – 25 August 1867) was an English scientist who contributed to the fields of electromagnetism and electrochemistry.
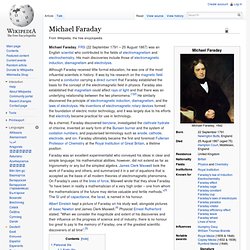
His main discoveries include those of electromagnetic induction, diamagnetism and electrolysis. As a chemist, Faraday discovered benzene, investigated the clathrate hydrate of chlorine, invented an early form of the Bunsen burner and the system of oxidation numbers, and popularised terminology such as anode, cathode, electrode, and ion. Faraday ultimately became the first and foremost Fullerian Professor of Chemistry at the Royal Institution of Great Britain, a lifetime position. Faraday was an excellent experimentalist who conveyed his ideas in clear and simple language; his mathematical abilities, however, did not extend as far as trigonometry or any but the simplest algebra. Johan August Arfwedson.
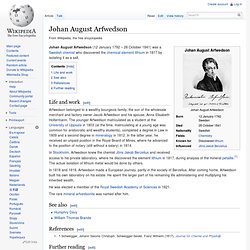
Johan August Arfwedson (12 January 1792 – 28 October 1841) was a Swedish chemist who discovered the chemical element lithium in 1817 by isolating it as a salt.
Joseph Black. Henry Cavendish. Henry Cavendish FRS (10 October 1731 – 24 February 1810) was a British natural philosopher, scientist, and an important experimental and theoretical chemist and physicist.
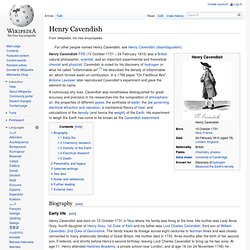
Cavendish is noted for his discovery of hydrogen or what he called "inflammable air".[1] He described the density of inflammable air, which formed water on combustion, in a 1766 paper "On Factitious Airs". Antoine Lavoisier later reproduced Cavendish's experiment and gave the element its name. Antoine Lavoisier. Antoine-Laurent de Lavoisier (also Antoine Lavoisier after the French Revolution; 26 August 1743 – 8 May 1794; French pronunciation: [ɑ̃twan lɔʁɑ̃ də lavwazje]) was a French nobleman and chemist central to the 18th-century Chemical Revolution and a large influence on both the histories of chemistry and biology.[1] He is widely considered to be the "Father of Modern Chemistry.
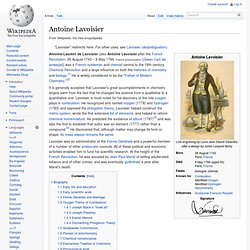
"[2] It is generally accepted that Lavoisier's great accomplishments in chemistry largely stem from the fact that he changed the science from a qualitative to a quantitative one. Lavoisier is most noted for his discovery of the role oxygen plays in combustion. Joseph Priestley. Joseph Priestley FRS (24 March [O.S. 13 March] 1733 – 6 February 1804) was an 18th-century English theologian, Dissenting clergyman, natural philosopher, chemist, educator, and political theorist who published over 150 works.
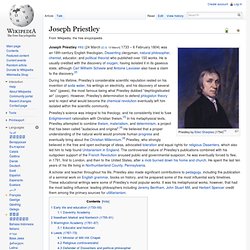
He is usually credited with the discovery of oxygen, having isolated it in its gaseous state, although Carl Wilhelm Scheele and Antoine Lavoisier also have a claim to the discovery.[2] During his lifetime, Priestley's considerable scientific reputation rested on his invention of soda water, his writings on electricity, and his discovery of several "airs" (gases), the most famous being what Priestley dubbed "dephlogisticated air" (oxygen).
However, Priestley's determination to defend phlogiston theory and to reject what would become the chemical revolution eventually left him isolated within the scientific community. Carl Wilhelm Scheele. Carl Wilhelm Scheele (9 December 1742 – 21 May 1786) was a Swedish Pomeranian pharmaceutical chemist.
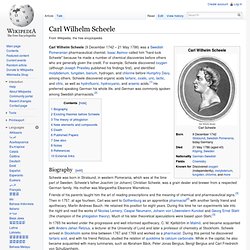
Isaac Asimov called him "hard-luck Scheele" because he made a number of chemical discoveries before others who are generally given the credit. Alessandro Volta. Early life and works[edit] Volta was born in Como, a town in present-day northern Italy (near the Swiss border) on February 18, 1745. In 1774, he became a professor of physics at the Royal School in Como. A year later, he improved and popularized the electrophorus, a device that produced static electricity. His promotion of it was so extensive that he is often credited with its invention, even though a machine operating on the same principle was described in 1762 by the Swedish experimenter Johan Wilcke.[3][4] In the years between 1776–78, Volta studied the chemistry of gases.
Mikhail Lomonosov. Mikhail Vasilyevich Lomonosov (Russian: Михаи́л Васи́льевич Ломоно́сов, IPA: [mʲɪxɐˈil vɐˈsʲilʲjɪvʲɪtɕ ləmɐˈnosəf] ( ); November 19 [O.S. November 8] 1711 – April 15 [O.S. John Dalton.