


Electromagnetic spectrum The electromagnetic spectrum is the range of all possible frequencies of electromagnetic radiation.[4] The "electromagnetic spectrum" of an object has a different meaning, and is instead the characteristic distribution of electromagnetic radiation emitted or absorbed by that particular object. Most parts of the electromagnetic spectrum are used in science for spectroscopic and other probing interactions, as ways to study and characterize matter.[6] In addition, radiation from various parts of the spectrum has found many other uses for communications and manufacturing (see electromagnetic radiation for more applications). History of electromagnetic spectrum discovery The first discovery of electromagnetic radiation other than visible light came in 1800, when William Herschel discovered infrared radiation.[7] He was studying the temperature of different colors by moving a thermometer through light split by a prism. Range of the spectrum where: Rationale for spectrum regional names Boundaries
Observable universe The surface of last scattering is the collection of points in space at the exact distance that photons from the time of photon decoupling just reach us today. These are the photons we detect today as cosmic microwave background radiation (CMBR). However, with future technology, it may be possible to observe the still older relic neutrino background, or even more distant events via gravitational waves (which also should move at the speed of light). Sometimes astrophysicists distinguish between the visible universe, which includes only signals emitted since recombination—and the observable universe, which includes signals since the beginning of the cosmological expansion (the Big Bang in traditional cosmology, the end of the inflationary epoch in modern cosmology). The Universe versus the observable universe[edit] If the Universe is finite but unbounded, it is also possible that the Universe is smaller than the observable universe. Size[edit] Misconceptions on its size[edit] Horizons[edit]
Ununoctium The radioactive ununoctium atom is very unstable, due to its high mass, and since 2005, only three or possibly four atoms of the isotope 294Uuo have been detected.[12] While this allowed for very little experimental characterization of its properties and possible compounds, theoretical calculations have resulted in many predictions, including some unexpected ones. For example, although ununoctium is a member of Group 18, it may possibly not be a noble gas, unlike all the other Group 18 elements.[1] It was formerly thought to be a gas but is now predicted to be a solid under normal conditions due to relativistic effects.[1] History[edit] Unsuccessful synthesis attempts[edit] In late 1998, Polish physicist Robert Smolańczuk published calculations on the fusion of atomic nuclei towards the synthesis of superheavy atoms, including ununoctium.[13] His calculations suggested that it might be possible to make ununoctium by fusing lead with krypton under carefully controlled conditions.[13]
Black body As the temperature of a black body decreases, its intensity also decreases and its peak moves to longer wavelengths. Shown for comparison is the classical Rayleigh–Jeans law and its ultraviolet catastrophe. A black body is an idealized physical body that absorbs all incident electromagnetic radiation, regardless of frequency or angle of incidence. A black body in thermal equilibrium (that is, at a constant temperature) emits electromagnetic radiation called black-body radiation. The radiation is emitted according to Planck's law, meaning that it has a spectrum that is determined by the temperature alone (see figure at right), not by the body's shape or composition. A black body in thermal equilibrium has two notable properties:[1] It is an ideal emitter: it emits as much or more energy at every frequency than any other body at the same temperature.It is a diffuse emitter: the energy is radiated isotropically, independent of direction. Definition[edit] Idealizations[edit] Realizations[edit]
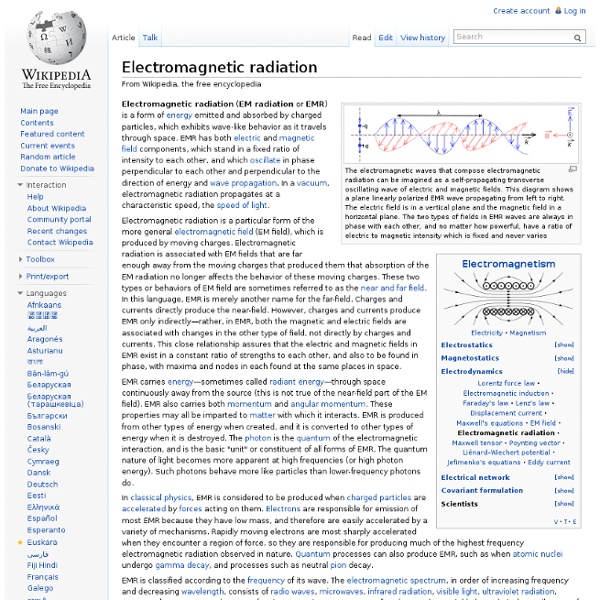
Electromagnetic field Electric and magnetic fields produced by moving charged objects The field can be viewed as the combination of an electric field and a magnetic field. The electric field is produced by stationary charges, and the magnetic field by moving charges (currents); these two are often described as the sources of the field. The way in which charges and currents interact with the electromagnetic field is described by Maxwell's equations and the Lorentz force law.[2] A sinusoidal electromagnetic wave propagating along the positive z-axis, showing the electric field (blue) and magnetic field (red) vectors. Structure[edit] The electromagnetic field may be viewed in two distinct ways: a continuous structure or a discrete structure. Continuous structure[edit] Classically, electric and magnetic fields are thought of as being produced by smooth motions of charged objects. Discrete structure[edit] The electromagnetic field may be thought of in a more 'coarse' way. Dynamics[edit] Feedback loop[edit] Gauss's law
Electron History[edit] In the early 1700s, Francis Hauksbee and French chemist Charles François de Fay independently discovered what they believed were two kinds of frictional electricity—one generated from rubbing glass, the other from rubbing resin. From this, Du Fay theorized that electricity consists of two electrical fluids, vitreous and resinous, that are separated by friction, and that neutralize each other when combined.[17] A decade later Benjamin Franklin proposed that electricity was not from different types of electrical fluid, but the same electrical fluid under different pressures. He gave them the modern charge nomenclature of positive and negative respectively.[18] Franklin thought of the charge carrier as being positive, but he did not correctly identify which situation was a surplus of the charge carrier, and which situation was a deficit.[19] Discovery[edit] A beam of electrons deflected in a circle by a magnetic field[25] Robert Millikan Atomic theory[edit]
Absolute zero Absolute zero is the lower limit of the thermodynamic temperature scale, a ficticious state at which the enthalpy and entropy of a cooled ideal gas reaches its minimum value, taken as 0. The theoretical temperature is determined by extrapolating the ideal gas law; by international agreement, absolute zero is taken as −273.15° on the Celsius scale (International System of Units),[1][2] which equates to −459.67° on the Fahrenheit scale (English/United States customary units).[3] The corresponding Kelvin and Rankine temperature scales set their zero points at absolute zero by definition. The laws of thermodynamics dictate that absolute zero cannot be reached using only thermodynamic means,[clarification needed] as the temperature of the substance being cooled approaches the temperature of the cooling agent asymptotically. A system at absolute zero still possesses quantum mechanical zero-point energy, the energy of its ground state. The kinetic energy of the ground state cannot be removed.
Energy Property that makes changes possible Common forms of energy include the kinetic energy of a moving object, the potential energy stored by an object (for instance due to its position in a field), the elastic energy stored in a solid object, chemical energy associated with chemical reactions, the radiant energy carried by electromagnetic radiation, and the internal energy contained within a thermodynamic system. All living organisms constantly take in and release energy. Due to mass–energy equivalence, any object that has mass when stationary (called rest mass) also has an equivalent amount of energy whose form is called rest energy, and any additional energy (of any form) acquired by the object above that rest energy will increase the object's total mass just as it increases its total energy. Human civilization requires energy to function, which it gets from energy resources such as fossil fuels, nuclear fuel, or renewable energy. Forms History Units of measure Scientific use Chemistry Biology
Frequently Asked Questions in Cosmology Tutorial : Part 1 | Part 2 | Part 3 | Part 4 | Age | Distances | Bibliography | Relativity What is the currently most accepted model for the Universe? The current best fit model is a flat ΛCDM Big Bang model where the expansion of the Universe is accelerating, and the age of the Universe is 13.7 billion years. Back to top. What is the evidence for the Big Bang? The evidence for the Big Bang comes from many pieces of observational data that are consistent with the Big Bang. The darkness of the night sky - Olbers' paradox. Why do we think that the expansion of the Universe is accelerating? The evidence for an accelerating expansion comes from observations of the brightness of distant supernovae. What is quintessence? Quintessence, or the fifth essence, is a fifth element beyond the standard earth, air, fire and water of ancient chemistry. If the Universe is only 14 billion years old, why isn't the most distant object we can see 7 billion light years away? What is the redshift?
Thermal radiation This diagram shows how the peak wavelength and total radiated amount vary with temperature according to Wien's displacement law. Although this plot shows relatively high temperatures, the same relationships hold true for any temperature down to absolute zero. Visible light is between 380 and 750 nm. Thermal radiation in visible light can be seen on this hot metalwork. Thermal radiation is electromagnetic radiation generated by the thermal motion of charged particles in matter. Examples of thermal radiation include the visible light and infrared light emitted by an incandescent light bulb, the infrared radiation emitted by animals and detectable with an infrared camera, and the cosmic microwave background radiation. If a radiation-emitting object meets the physical characteristics of a black body in thermodynamic equilibrium, the radiation is called blackbody radiation.[1] Planck's law describes the spectrum of blackbody radiation, which depends only on the object's temperature. Here,
Gravity Attraction of masses and energy In physics, gravity (from Latin gravitas 'weight'[1]) is a fundamental interaction which causes mutual attraction between all things that have mass. Gravity is, by far, the weakest of the four fundamental interactions, approximately 1038 times weaker than the strong interaction, 1036 times weaker than the electromagnetic force and 1029 times weaker than the weak interaction. As a result, it has no significant influence at the level of subatomic particles.[2] However, gravity is the most significant interaction between objects at the macroscopic scale, and it determines the motion of planets, stars, galaxies, and even light. On Earth, gravity gives weight to physical objects, and the Moon's gravity is responsible for sublunar tides in the oceans (the corresponding antipodal tide is caused by the inertia of the Earth and Moon orbiting one another). Definitions History Ancient world In the ancient Middle East, gravity was a topic of fierce debate. Specifics
Radiation Illustration of the relative abilities of three different types of ionizing radiation to penetrate solid matter. Typical alpha particles (α) are stopped by a sheet of paper, while beta particles (β) are stopped by an aluminium plate. Gamma radiation (γ) is damped when it penetrates lead. In electromagnetic radiation (such as microwaves from an antenna, shown here) the term "radiation" applies only to the parts of the electromagnetic field that radiate into infinite space and decrease in intensity by an inverse-square law of power so that the total radiation energy that crosses through an imaginary spherical surface is the same, no matter how far away from the antenna the spherical surface is drawn. Gamma rays, x-rays and the higher energy range of ultraviolet light constitute the ionizing part of the electromagnetic spectrum. The word radiation arises from the phenomenon of waves radiating (i.e., traveling outward in all directions) from a source. Ionizing radiation[edit] X-ray[edit]