

Blog SEFAP Hidroxicloroquina y su posible efecto protector en pacientes en tratamiento crónico. BMJ 02.03 HCQ Prevención Covid. This is a living guideline, so the recommendations included here will be updated, and new recommendations will be added for other prophylactic drugs for covid-19.
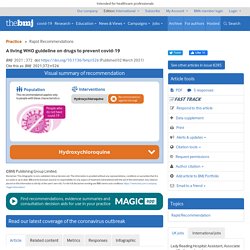
The infographic provides a summary of the recommendations and includes links to the MAGICapp for more details on the evidence and rationale for the recommendation, as well as patient decision aids. Box 2 outlines key methodological aspects of the guideline process. Box 2 How this living guideline was created (see MAGICapp for full details This guideline was developed by WHO and the MAGIC Evidence Ecosystem Foundation (MAGIC) with support from The BMJ.
Standards, methods, and processes for living and trustworthy guidance Selection and support of the guideline development panel As recommended by the WHO handbook, the panel aimed to create a recommendation based on consensus but elected, at the beginning of the first panel meeting, to call a vote if a consensus could not be reached. Sources of evidence. MPR 11.11 NIH Hydroxychloroquine Not Effective for COVID 19. Why Are We Still Talking About Hydroxychloroquine as a Treatment for COVID-19? [Skip to Content] [Skip to Content Landing] Claim CME Share Facebook Twitter LinkedIn Email Copy URL Critical Care Medicine.
Jama saag 09.11 Misguided use of HCQ Editorial. Jama self 09.11 HCQ EC 14 d hospitalizados. Nejm 08.10 HCQ. Jamainternal abella 30.09 HCQ profilaxis. Medscape 28.09 Nueva mirada a la seguridad de HCQ. Medscape 07.08.20 HCQ intervalo QT. Ann Intern Med 30.07 HCQ y COVID. HCQ een fases iniciales visual abstract Ann Intern Med. 16.07 Ann Intern Med HCQ en fases iniciales COVID EC. 16.07 Ann Intern Med The saga of HCQ: a cautionary tale Ed. MPR 10 jul ACP no recomienda HCQ. HCQ positivo 29.96 Int J Infec Dis. Jama rosenberg 11.05 HCQ y Azitro y mortalidad NY. 20200608 NOTA INFORMATIVA DAN HCQ Lancet y NEJM. BMJ 14.05 HCQ EC abierto leve a mooderado. BMJ 19.05 Falta de eficacia HCQ ed. NEJM 03.06 HCQ prevención postexposición. Mandeep 22.05.2020 Observacional Clorq HCQ con macrolido Lancet.
22.05 Toxicidad CV HCQ y azitromicina. Base FV OMS CIRCULATION. 01.05 Awadhesh RS y MA Hidroxicloroquina (1) Macias Incidencia similar COVID ptes reumatología con y sin HCQ. Mayo 2020 Awadhesh RS y MA Hidroxicloroquina. 13.05 NEJM Journal Watch Carencia eficacia HCQ. JAMA rosenberg 11.05. HCQ y mortalidad. NEJM Geleris 07.05 HCQ observacional. Medscape 08.04 ISAC en contra primer estudio fav HCQ. Intern J Rheum Dis 13.04 RS HCQ prev.
COVID-19: reminder of risk of serious side effects with chloroquine and hydroxychloroquine. Chloroquine and hydroxychloroquine are known to potentially cause heart rhythm problems, and these could be exacerbated if treatment is combined with other medicines, such as the antibiotic azithromycin, that have similar effects on the heart.

Recent studies1,2 have reported serious, in some cases fatal, heart rhythm problems with chloroquine or hydroxychloroquine, particularly when taken at high doses or in combination with the antibiotic azithromycin. Chloroquine and hydroxychloroquine are currently authorised for treating malaria and certain autoimmune diseases. In addition to side effects affecting the heart, they are known to potentially cause liver and kidney problems, nerve cell damage that can lead to seizures (fits) and low blood sugar (hypoglycaemia). These medicines are being used in the context of the ongoing pandemic for treating patients with COVID-19 and investigated in clinical trials. JAMA Newt Open 24.04 Alta vs baja dosis CQ. MPR 30.04 Cloroquina altas dosis COVID. Medscape 28.04 Cambio protocolos hospital tras alerta FDA HCQ. Jamainternal 29.04 HCQ No dañar. MEJM Journal Watch 24.04 HCQ old drugs new world. CMAJ 27.04 Seguridad HCQ. Informecloroquina hidroxicloroquinaparaCOVID 19270.
CEBM Oxford 14.04Hydroxychloroquine for COVID 19 What do the clinical trials tell us. CADTH 20.04 CQ o HCQ con o sin AZITRO for COVID 19. NEJM Journal Watch 24.04 HCQ poca eficacia Comentario. FDA Alerta HCQ. JAMA 24.04 Ed. Seguridad HCQ. JAMA 24.04 EC CQ en COVID grave. Search of: Hydroxychloroquine. A type of eligibility criteria that indicates whether people who do not have the condition/disease being studied can participate in that clinical study.An arm type in which a group of participants receives an intervention/treatment considered to be effective (or active) by health care providers.An unfavorable change in the health of a participant, including abnormal laboratory findings, that happens during a clinical study or within a certain amount of time after the study has ended.
This change may or may not be caused by the intervention/treatment being studied.A type of eligibility criteria that indicates the age a person must be to participate in a clinical study. This may be indicated by a specific age or the following age groups:The age groups are: Child (birth-17)Adult (18-64)Older Adult (65+)A measure of all deaths, due to any cause, that occur during a clinical study.A method used to assign participants to an arm of a clinical study.
MPR 22.04 FDA nuevo ensayo HCQ. Pastick Rev. Cloroquina e Hidroxicloroquina. 20.05 Macias Incidencia similar COVID ptes reumatología con y sin HCQ. MPR 14.04 estudio con Cloroquina interrumpido por aumento de muertes. 23.03 Ontario Pharm Statement CQ HCQ v2. MedRxiv 10.04 HCQ no eficaz en ingresadoss con oxigenotp. Gautret HCQ y AZITROMICINA. Abierto no randomizado 20 04. SACYL COViD 19 HIDROXICLOROQUINA Y AZITROMICINA. MPR 08.04 HCQ rev. Medscape AZ HCQ cuidado QT. NIH clinical trial of hydroxychloroquine ORCHID begins. Loading Skip to main content Site Menu COVID-19 is an emerging, rapidly evolving situation.
Get the latest public health information from CDC: Get the latest research information from NIH: You are here Home » News & Events » News Releases News Releases News Release Thursday, April 9, 2020 NIH clinical trial of hydroxychloroquine, a potential therapy for COVID-19, begins AddThis Sharing Buttons Share to PrintShare to EmailShare to FacebookShare to TwitterShare to More Colorized scanning electron micrograph of an apoptotic cell (green) heavily infected with SARS-COV-2 virus particles (purple), isolated from a patient sample. A clinical trial to evaluate the safety and effectiveness of hydroxychloroquine for the treatment of adults hospitalized with coronavirus disease 2019 (COVID-19) has begun, with the first participants now enrolled in Tennessee.
The first participants have enrolled in the trial at Vanderbilt University Medical Center, Nashville, one of dozens of centers in the PETAL Network. MPR 10.04 NIH Trial Evaluating Hydroxychloroquine for COVID 19 ORCHID Underway. Can Pharm Ass 23.03. CQ HCQ COVID FINAL. Ann Intern Med HCQ lo que cuaquier médico debería saber 31. Ann Med Intern HCQ a rush to judgment 30. Medscape HCQ ¿Cuáles son los datos? Chloroquine and hydroxychloroquine for coronavirus Cochrane COVID Rapid Reviews. HCQ EC China. BITn Informe cloroquina hidroxicloroquina.
MPR FDA Studies Underway Chloroquine. Coronavirus (COVID-19) Update: Chloroquine/Hydroxychloroquine and Azithromycin. [Skip to Content] [Skip to Content Landing] Claim CME Share Facebook Twitter LinkedIn Email Copy URL Clinical Pharmacy and Pharmacology Coronavirus (COVID-19) Update: Chloroquine/Hydroxychloroquine and Azithromycin Educational ObjectiveReview update on risks and benefits of Chloroquine/Hydroxychloroquine and Azithromycin in treating COVID-19 0.25 Credit CME March 24, 2020 Clinical Review Audio 17 min 13 sec MOC/CME Information Listen Claim CME Coronavirus (COVID-19) Update: Chloroquine/Hydroxychloroquine and Azithromycin Download MP3 Subscribe to Podcast Claim CME Sign in to take quiz and track your certificates Sign in JN Learning™ is the home for CME and MOC from the JAMA Network.
Related Treating COVID-19—Off-Label and Compassionate Use and Clinical Trials During Pandemics 1.00 CME You May Also Like Clinical Pharmacy and Pharmacology 35m 58s 0.5 Credit CME Coronavirus in New York - Report From the Front Lines Clinical Pharmacy and Pharmacology Global Health JAMA Ophthalmology See More About Button To Top. FDA MedWatch Chloroquine Phosphate Intende.. BMJ 08.04 HCQ y CQ Ed.