

Diana's Tree. Well-developed Diana's tree grown up over copper rod from silver/mercury amalgam placed in 0.1 M solution of silver nitrate - reaction time 2 hrs.

In pre-modern chemistry, the various methods for procuring Diana's Tree were exceedingly time-consuming; for example, the following process, originally described by Nicolas Lemery, required forty days to see results: "Dissolve an ounce of pure silver in a sufficient quantity of aqua fortis, exceedingly pure, and of a moderate strength, and having put the solution in a jar, dilute it with about twenty ounces of distilled water.
Then add two ounces of mercury, and leave the whole at rest. In the course of forty days, there will rise from the mercury a kind of tree, which throwing out branches will represent natural vegetation. The form of this metallic tree may be varied as desired. References[edit] ^ Jump up to: a b Brewer, E. External links[edit] "Arbor Dianæ". Simple reaction makes the building blocks of a nucleic acid. Origin-of-life researchers face a deceptively straightforward question: how did simple chemicals produce complex biochemistry?
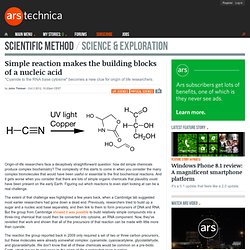
The complexity of this starts to come in when you consider the many complex biomolecules that would have been useful or essential to the first biochemical reactions. And it gets worse when you consider that there are lots of simple organic chemicals that plausibly could have been present on the early Earth. Figuring out which reactions to even start looking at can be a real challenge. The extent of that challenge was highlighted a few years back, when a Cambridge lab suggested most earlier researchers had gone down a dead end. Previously, researchers tried to build up a sugar and a nucleic acid base separately, and then link to them to form precursors of DNA and RNA. In a new paper, the same lab tackles forming the simple, two- and three-atom sugars used in their earlier work (glycolaldehyde and glyceraldehyde).
ChemReference: Periodic Table and Chemistry Reference. Linking simple chemistry to something like life. Origin of life researchers have made impressive progress in recent years, showing that simple chemicals can combine to make nucleotides, the building blocks of DNA and RNA.
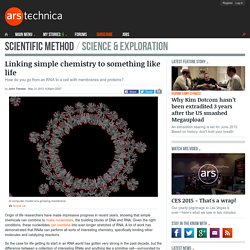
Given the right conditions, these nucleotides can combine into ever-longer stretches of RNA. A lot of work has demonstrated that RNAs can perform all sorts of interesting chemistry, specifically binding other molecules and catalyzing reactions. So the case for life getting its start in an RNA world has gotten very strong in the past decade, but the difference between a collection of interesting RNAs and anything like a primitive cell—surrounded by membranes, filled with both RNA and proteins, and running a simple metabolism—remains a very wide chasm. Or so it seems. A set of papers that came out in the past several days suggest that the chasm might not be as large as we'd tend to think. Ironing out metabolism DNA and RNA tend to have nothing to do with iron, interacting with magnesium instead.
Proteins build membranes. All the elements arranged by the country that discovered them. 05/09 > BE Belgique 61 > Recherches à l'ULB : un cadre théorique pour comprendre les effets de la discontinuité moléculaire. BiologieRecherches à l'ULB : un cadre théorique pour comprendre les effets de la discontinuité moléculaire La discontinuité moléculaire est apparente dans des systèmes chimiques de petit volume et conduit à des cinétiques stochastiques.

L'article paru dans Nature Communications expose le travail de Nélido González-Segredo, et de ses collaborateurs de l'Institut d'Informatique Théorique de l'ETH de Zurich et de la Faculté des Sciences Biologiques de l'Université d'Edimbourg. Ils y présentent un cadre théorique pour comprendre les effets de la discontinuité sur l'état stationnaire d'un réseau de réactions monostables. Ils examinent différentes solutions de la même concentration dans des compartiments de différents volumes. Les équations cinétiques classiques ignorent la discontinuité et prévoient les mêmes concentrations moyennes dans tous les compartiments. 03/19 > BE Espagne 113 > A la recherche d'une meilleure productivité des énergies renouvelables. Lead (Pb) Periodic Table Software - The Elements and Isotopes - Download Spectra Program. Impossible reactions: Five chemistry rules broken.
Cookies on the New Scientist website close Our website uses cookies, which are small text files that are widely used in order to make websites work more effectively.
To continue using our website and consent to the use of cookies, click away from this box or click 'Close' Find out about our cookies and how to change them. Impossible chemistry: Crystal paradox - physics-math - 23 January 2012. When Dan Shechtman said he had found "quasicrystalline" atomic symmetry, a Nobel laureate ridiculed him.
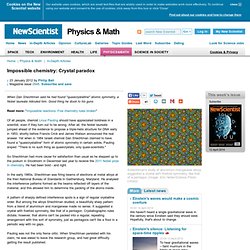
Good thing he stuck to his guns. Impossible chemistry: Having entropy both ways - 23 January 2012. A reaction that went spontaneously in both directions seemed to break one of science's most cherished laws – only a crank would say they'd made it happen Read more: "Impossible reactions: Five chemistry rules broken" For Boris Belousov, vindication came too late.
When the Soviet biochemist was awarded his country's prestigious Lenin prize in 1980, he had been dead for 10 years. At least he lived long enough to see the scorn heaped on his work give way to grudging acceptance. In the 1950s, he had devised a cocktail of chemical ingredients that provided a simple analogue of glycolysis, the process by which enzymes break down sugars. But then something astonishing happened: the cocktail went colourless again. This was unacceptable.