

A Vaxxing Question. Suzie Halewood Dr Frances Kelsey receiving the President’s Award for Distinguished Federal Civilian Service from President Kennedy in 1962, for successfully preventing Thalidomide being approved for use in the USA.
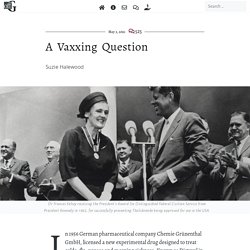
In 1956 German pharmaceutical company Chemie Grünenthal GmbH, licensed a new experimental drug designed to treat colds, flu, nausea and morning sickness. Known as Distaval in the UK, Distillers Biochemicals Ltd declared the drug could ‘be given with complete safety to pregnant women and nursing mothers without adverse effect on mother or child’ – a basic pre-requisite for licensing a drug. While forty-nine countries licensed the drug under multiple different names, the then head of the FDA Dr. Covid-19: Workers at U.S. Meat Plants Now Have Vaccine Access in Most States. Employees at food processing facilities, which had some of the country’s largest known coronavirus outbreaks early in the pandemic, are now eligible for vaccines in at least 26 states, a New York Times survey found.

The expansion of vaccines to food processing workers comes amid rapid widening of eligibility, especially for essential workers at greater risk of contracting the virus. Almost every state is vaccinating some subset of frontline workers, but the list of eligible professions varies widely. In at least six states, food processing workers are eligible in certain counties but not in others. Is Russia’s COVID-19 vaccine safe? Brazil’s veto of Sputnik V sparks lawsuit threat and confusion.
Science’s COVID-19 reporting is supported by the Heising-Simons Foundation.
A confusing and unusually nasty fight broke out this week over the safety of a Russian COVID-19 vaccine known as Sputnik V after a Brazilian health agency declined on Monday to authorize its import because of quality and safety concerns. The stakes escalated yesterday when the Twitter account officially associated with the vaccine said “Sputnik V is undertaking a legal defamation proceeding” against Brazil’s regulators. In an online press conference several hours later, the Brazilian Health Regulatory Agency (Anvisa) defended its decision, maintaining that documentation from some of the Russian facilities making Sputnik V shows that one of its two doses contains adenoviruses capable of replication, a potential danger to vaccine recipients. The vaccine uses two different adenoviruses, which cause the common cold, to deliver the gene for the spike protein of SARS-CoV-2, the virus that causes COVD-19.
Related. Why Covid Vaccine Testing Is a Farce. The flaws of vaccine trials in general are really highlighted by current COVID-19 vaccine studies, one of the most egregious ones being the fact that vaccine makers rarely use inert placebos (such as a saline shot), which is the gold standard for drug trials.

As noted in a January 25, 2021, article in The Defender,1 vaccine developers typically assess the safety of a new vaccine against another vaccine, and by so doing, they effectively hide side effects as most vaccines have side effects and risks. As just one example, the Oxford/AstraZeneca COVID-19 vaccine is being tested against a meningitis vaccine,2 which just so happens to share many of the side effects reported from COVID-19 vaccines. As reported by the National Vaccine Information Center:3 “According to the CDC, at least 50% of individuals receiving meningococcal vaccines targeting meningococcal serogroups A, C, Y, and W-135 (Menactra or Menveo) experience mild side effects …
How NPR Taught Me to Worry About the Police and Trust the Jab. I am a social scientist who has written a little bit on media over the years, so sometimes, in the spirit of research, when I have a few extra minutes in the car, I put on the radio.
In just 15 minutes or so this morning I gathered the following information. I heard this segment on the serious blood clots that some vaccinated with the Johnson & Johnson COVID-19 vaccine have suffered. Note how NPR interviewer, Steve Inskeep, works so assiduously to insinuate it is absolutely insane that anyone would be worried about the chances of an adverse reaction to the vaccine. After all, it was, he says, “literally a one-in-a-million event.” British Variant 45% More Contagious than Original Virus. A new study at Tel Aviv University found that the British variant (termed: B.1.1.7) of Covid-19 is 45% more contagious than the original virus.

The researchers relied on data from about 300,000 PCR tests for Covid-19 obtained from the COVID-19 testing lab, which was established in collaboration with the Electra Group. According to the researchers, “The study proves that active monitoring of at-risk populations and prioritized vaccination programs can prevent hundreds of deaths.” V-safe After Vaccination Health Checker. Clinical Immunization Safety Assessment (CISA) Project. Healthcare providers or health departments in the United States can request a consultation from CISA COVIDvax for a complex COVID-19 vaccine safety question that is (1) about an individual patient residing in the United States or vaccine safety issue and (2) not readily addressed by CDC or Advisory Committee on Immunization Practices (ACIP) guidelines.
This request can be made through CDC-INFO by: Calling 800-CDC-INFO (800-232-4636), orSubmitting a request via CDC-INFO webform. Interim Clinical Considerations for Use of COVID-19 Vaccines. Background The Advisory Committee on Immunization Practices (ACIP) has issued interim recommendations for the use of Pfizer-BioNTech, Moderna, and Janssen (Johnson & Johnson) COVID-19 vaccines for the prevention of coronavirus disease 2019 (COVID-19) in the United States.

On April 23, 2021, ACIP reaffirmed its interim recommendation for use of the Janssen COVID-19 vaccine in all persons aged ≥18 years under the Food and Drug Administration’s (FDA) Emergency Use Authorization (EUA). ACIP’s updated recommendation followed a review of cases of thrombosis with thrombocytopenia syndrome (TTS) among Janssen COVID-19 vaccine recipients and consideration of the risks and benefits of resuming vaccination. FDA has added a warning to the Janssen COVID-19 vaccine EUAexternal icon fact sheets and prescribing information that rare clotting events might occur after vaccination, primarily among women aged 18–49 years.
Une seule dose de vaccin pour les personnes ayant déjà été infectées par le SARS-CoV-2. Stratégie de vaccination contre le SARS-CoV-2 - Vaccination des personnes ayant un antécédent de Covid-19. Strategie de vaccination contre le sars cov 2 vaccination des personnes ayant un antecedent de covid 19 synthese. Characterization of pre-existing and induced SARS-CoV-2-specific CD8 + T cells. Study cohort A total of 26 convalescent individuals (following a mild course of SARS-CoV-2 infection) and 25 age- and sex-matched historic controls (collected before August 2019) of healthy individuals (including pre-infection samples of longitudinal cases) were recruited at the Freiburg University Medical Center, Germany.

A mild course of infection was defined as clinical symptoms without signs of respiratory insufficiency. The donor characteristics are summarized in Supplementary Table 2. SARS-CoV-2 infection was confirmed by positive PCR testing from oropharyngeal swab and/or SARS-CoV-2 spike IgG positive antibody testing in the presence of typical symptoms. pMHCI tetramer-based magnetic bead enrichment of virus-specific CD8+ T cells was performed with samples from 18 SARS-CoV-2 convalescent individuals and 10 historic controls. HLA typing was performed by next-generation sequencing and is presented in Supplementary Table 2. Vaccine Adverse Event Reporting System (VAERS) VAERS - Information for Healthcare Providers. Management of Anaphylaxis at COVID-19 Vaccination Sites. Summary of recent changes (last updated March 3, 2021) Considerations broadened to include use of Janssen (Johnson & Johnson) COVID-19 vaccine.
Key Points Under the Emergency Use Authorizationsexternal icon for COVID-19 vaccines, appropriate medical treatment for severe allergic reactions must be immediately available in the event that an acute anaphylactic reaction occurs following administration of a COVID-19 vaccine. These interim considerations provide information on preparing for the initial assessment and management of anaphylaxis following COVID-19 vaccination. Lab Testing After Severe Allergic Reaction Following COVID-19 Vaccination. There are no specific lab tests that can definitively diagnose the cause of a severe allergic reaction (e.g., anaphylaxis) following COVID-19 vaccination.
In the United States, two commercially available lab tests can be ordered by healthcare providers and processed through healthcare facilities to better characterize a severe allergic reaction. This document provides an overview of the timing and procedure for collecting blood samples for these lab tests. These samples should only be collected after medically stabilizing a patient who has experienced a severe allergic reaction.
The two commercially available lab tests are: How the Pfizer-BioNTech Covid-19 Vaccine Works. How Moderna’s Covid-19 Vaccine Works. The First Dose is Good. WSJ: The Covid-19 vaccine developed by Pfizer Inc. and BioNTech SE generates robust immunity after one dose and can be stored in ordinary freezers instead of at ultracold temperatures, according to new research and data released by the companies.The findings provide strong arguments in favor of delaying the second dose of the two-shot vaccine, as the U.K. has done . They could also have substantial implications on vaccine policy and distribution around the world, simplifying the logistics of distributing the vaccine.A single shot of the vaccine is 85% effective in preventing symptomatic disease 15 to 28 days after being administered, according to a peer-reviewed study conducted by the Israeli government-owned Sheba Medical Center and published in the Lancet medical journal.
It’s becoming clearer that delaying the second dose is the right strategy but it was the right strategy back in December when I first started advocating for First Doses First. FDA Advisory Panel Takes Up Pfizer-BioNTech Vaccine. By and The Food and Drug Administration and other federal agencies will monitor the use of Covid-19 vaccines long after their release, including the question of how long vaccines will confer immunity, federal officials said at an FDA vaccine panel meeting Thursday. , a senior official at the U.S. Centers for Disease Control and Prevention specializing in vaccines, said her agency, along with the FDA and the Department of Defense, will conduct "active surveillance" of health care workers and residents of long-term care facilities to gauge the effects of the immunization. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Structure of the nCoV trimeric spike The World Health Organization has declared the outbreak of a novel coronavirus (2019-nCoV) to be a public health emergency of international concern.
The virus binds to host cells through its trimeric spike glycoprotein, making this protein a key target for potential therapies and diagnostics. Wrapp et al. determined a 3.5-angstrom-resolution structure of the 2019-nCoV trimeric spike protein by cryo–electron microscopy. Using biophysical assays, the authors show that this protein binds at least 10 times more tightly than the corresponding spike protein of severe acute respiratory syndrome (SARS)–CoV to their common host cell receptor. The race for coronavirus vaccines: a graphical guide. More than 90 vaccines are being developed against SARS-CoV-2 by research teams in companies and universities across the world. Researchers are trialling different technologies, some of which haven’t been used in a licensed vaccine before. At least six groups have already begun injecting formulations into volunteers in safety trials; others have started testing in animals.
Playability. Moderna’s top scientist: ‘We are actually hacking the software of life’ – LeoHohmann.com. Tucker Carlson: Public confidence in the coronavirus vaccine won't be achieved by Big Tech censorship. Here's Why Some Health Care Workers Don't Want The COVID-19 Vaccine. Delay Second Doses? A Guide To The Latest COVID-19 Vaccine Debate. Interventionally. Immunity. Vaccine Flux.