Covid-19 Vaccine
>
Doleole
>
La toile du vivant
>
The Web of Life
>
Intelligence
>
Existential Intelligence
>
Sciences
>
Natural Science
>
Life Sciences
>
Biology
>
Microbiology
>
Virus
>
Pandemic
>
Covid-19
Covid-19: Oxford-AstraZeneca vaccine approved for use in UK. 'Incredible milestone for science.' Pfizer and BioNTech update their promising COVID-19 vaccine result. Science’s COVID-19 reporting is supported by the Pulitzer Center and the Heising-Simons Foundation.
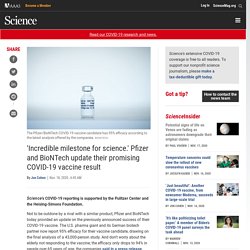
Not to be outdone by a rival with a similar product, Pfizer and BioNTech today provided an update on the previously announced success of their COVID-19 vaccine. The U.S. pharma giant and its German biotech partner now report 95% efficacy for their vaccine candidate, drawing on the final analysis of a 43,000-person study. And don’t worry about the elderly not responding to the vaccine; the efficacy only drops to 94% in people over 65 years of age, the companies said in a press release. As opposed to the vague initial report last week that their vaccine had greater than 90% efficacy, Pfizer and BioNTech are providing more specific data now that the study has reached enough COVID-19 cases to end. In all, the trial had 162 confirmed cases of symptomatic COVID-19 in the placebo group versus eight among those who received the two scheduled doses of the vaccine.
Pfizer's CEO cashed out 60% of his stock on the same day the company unveiled the results of its COVID-19 vaccine trial. Pfizer CEO Albert Bourla sold 62% of his stock in the company on the same day the drugmaker announced the results of its COVID-19 vaccine trial.Bourla sold $5.6 million in stock on Monday as part of a predetermined trading plan adopted August 19.His stock sale was carried out at $41.94 a share.
The 52-week-high for Pfizer stock is $41.99, which means the CEO cashed out his shares at close to their highest price this year.Pfizer and its German partner BioNTech on Monday became the first to post positive results from late-stage COVID-19 vaccine trials.Visit Business Insider's homepage for more stories. Pfizer CEO Albert Bourla sold 62% of his stock on the same day the company announced its experimental COVID-19 vaccine succeeded in clinical trials. The vaccine announcement sent Pfizer's shares soaring almost 15% on the day. "Through our stock plan administrator, Dr. Read more: We just got our first evidence that a coronavirus vaccine works. "Through our stock plan administrator, Dr.
Pfizer and BioNTech Announce Vaccine Candidate Against COVID-19 Achieved Success in First Interim Analysis from Phase 3 Study. Vaccine candidate was found to be more than 90% effective in preventing COVID-19 in participants without evidence of prior SARS-CoV-2 infection in the first interim efficacy analysisAnalysis evaluated 94 confirmed cases of COVID-19 in trial participants Study enrolled 43,538 participants, with 42% having diverse backgrounds, and no serious safety concerns have been observed; Safety and additional efficacy data continue to be collected Submission for Emergency Use Authorization (EUA) to the U.S.

Food and Drug Administration (FDA) planned for soon after the required safety milestone is achieved, which is currently expected to occur in the third week of November Clinical trial to continue through to final analysis at 164 confirmed cases in order to collect further data and characterize the vaccine candidate’s performance against other study endpoints This press release features multimedia. View the full release here: NEW YORK & MAINZ, GERMANY--(BUSINESS WIRE)-- Pfizer Inc.
COVID Update May 7. A recent Nature paper reveal a remarkable trick SARS-Cov-2 learned. A thread on #SARSCoV2 mutations and what they might mean for the #COVID19 vaccination and immunity. AI could help with the next pandemic—but not with this one.
It was an AI that first saw it coming, or so the story goes.
On December 30, an artificial-intelligence company called BlueDot, which uses machine learning to monitor outbreaks of infectious diseases around the world, alerted clients—including various governments, hospitals, and businesses—to an unusual bump in pneumonia cases in Wuhan, China. It would be another nine days before the World Health Organization officially flagged what we’ve all come to know as Covid-19. BlueDot wasn’t alone. An automated service called HealthMap at Boston Children’s Hospital also caught those first signs.
As did a model run by Metabiota, based in San Francisco. You can read all of our coverage of the coronavirus/Covid-19 outbreak for free, and also sign up for our coronavirus newsletter. But how much has AI really helped in tackling the current outbreak? The hype outstrips the reality. So here’s a reality check: AI will not save us from the coronavirus—certainly not this time. Prediction Early diagnosis. Coronavirus Vaccine Tracker. COVID-19 vaccine. Hypothetical vaccine against COVID-19 A COVID-19 vaccine is a hypothetical vaccine against coronavirus disease 2019 (COVID‑19).
Although no vaccine has completed clinical trials, there are multiple attempts in progress to develop such a vaccine. By May, 159 vaccine candidates were in development, with five having been initiated in Phase I–II safety and efficacy studies in human subjects, and seven in Phase I trials.[6][7] Globally accelerated development Among organizations that have formed international alliances to expedite vaccine development and prepare for distribution are: Federal governments dedicating resources for national or international investments include: Partnerships and competition WHO Solidarity trial Adaptive design for the Solidarity trial Partnerships, competition, and distribution Compressed timelines Technology platforms.
Covid-19 – Living NMA – A living mapping on registered trials. Vaccine. Sanitary Crisis.





